+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4240 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Bact spliceosome state 8 unmasked | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpost-spliceosomal complex / RES complex / negative regulation of chemokine-mediated signaling pathway / snoRNA splicing / U11/U12 snRNP / regulation of retinoic acid receptor signaling pathway / post-mRNA release spliceosomal complex / 3'-5' RNA helicase activity / U2 snRNP binding / U7 snRNA binding ...post-spliceosomal complex / RES complex / negative regulation of chemokine-mediated signaling pathway / snoRNA splicing / U11/U12 snRNP / regulation of retinoic acid receptor signaling pathway / post-mRNA release spliceosomal complex / 3'-5' RNA helicase activity / U2 snRNP binding / U7 snRNA binding / histone pre-mRNA DCP binding / generation of catalytic spliceosome for first transesterification step / U7 snRNP / cis assembly of pre-catalytic spliceosome / histone pre-mRNA 3'end processing complex / regulation of vitamin D receptor signaling pathway / SLBP independent Processing of Histone Pre-mRNAs / SLBP Dependent Processing of Replication-Dependent Histone Pre-mRNAs / spliceosome conformational change to release U4 (or U4atac) and U1 (or U11) / B-WICH complex / miRNA processing / nuclear retinoic acid receptor binding / embryonic brain development / oocyte development / alternative mRNA splicing, via spliceosome / U12-type spliceosomal complex / protein methylation / poly(A) binding / 7-methylguanosine cap hypermethylation / U1 snRNP binding / RNA splicing, via transesterification reactions / U2-type catalytic step 1 spliceosome / methylosome / pre-mRNA binding / C2H2 zinc finger domain binding / pICln-Sm protein complex / positive regulation of mRNA splicing, via spliceosome / snRNP binding / mRNA 3'-end processing / regulation of mRNA splicing, via spliceosome / sno(s)RNA-containing ribonucleoprotein complex / blastocyst formation / small nuclear ribonucleoprotein complex / splicing factor binding / Notch binding / SMN-Sm protein complex / spliceosomal tri-snRNP complex / host-mediated activation of viral transcription / mRNA cis splicing, via spliceosome / U2-type precatalytic spliceosome / P granule / positive regulation of vitamin D receptor signaling pathway / commitment complex / telomerase holoenzyme complex / nuclear vitamin D receptor binding / U2-type prespliceosome assembly / U2-type spliceosomal complex / Regulation of gene expression in late stage (branching morphogenesis) pancreatic bud precursor cells / Transport of Mature mRNA derived from an Intron-Containing Transcript / telomerase RNA binding / RUNX3 regulates NOTCH signaling / U2-type catalytic step 2 spliceosome / NOTCH4 Intracellular Domain Regulates Transcription / SAGA complex / U2 snRNP / U1 snRNP / RNA Polymerase II Transcription Termination / RHOBTB1 GTPase cycle / U4 snRNP / NOTCH3 Intracellular Domain Regulates Transcription / positive regulation of neurogenesis / U2-type prespliceosome / positive regulation of transcription by RNA polymerase III / protein peptidyl-prolyl isomerization / K63-linked polyubiquitin modification-dependent protein binding / inner cell mass cell proliferation / ubiquitin-ubiquitin ligase activity / nuclear androgen receptor binding / cyclosporin A binding / precatalytic spliceosome / generation of catalytic spliceosome for second transesterification step / WD40-repeat domain binding / Notch-HLH transcription pathway / lipid biosynthetic process / Formation of paraxial mesoderm / positive regulation of transforming growth factor beta receptor signaling pathway / pattern recognition receptor activity / SMAD binding / regulation of RNA splicing / spliceosomal complex assembly / mRNA 3'-splice site recognition / positive regulation of transcription by RNA polymerase I / mRNA Splicing - Minor Pathway / spliceosomal tri-snRNP complex assembly / Prp19 complex / U5 snRNA binding / U5 snRNP / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / blastocyst development / protein K63-linked ubiquitination Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.5 Å | |||||||||
 Authors Authors | Haselbach D / Komarov I / Agafonov D / Kastner B / Luehrmann R / Stark H | |||||||||
 Citation Citation |  Journal: Cell / Year: 2018 Journal: Cell / Year: 2018Title: Structure and Conformational Dynamics of the Human Spliceosomal B Complex. Authors: David Haselbach / Ilya Komarov / Dmitry E Agafonov / Klaus Hartmuth / Benjamin Graf / Olexandr Dybkov / Henning Urlaub / Berthold Kastner / Reinhard Lührmann / Holger Stark /  Abstract: The spliceosome is a highly dynamic macromolecular complex that precisely excises introns from pre-mRNA. Here we report the cryo-EM 3D structure of the human B spliceosome at 3.4 Å resolution. In ...The spliceosome is a highly dynamic macromolecular complex that precisely excises introns from pre-mRNA. Here we report the cryo-EM 3D structure of the human B spliceosome at 3.4 Å resolution. In the B state, the spliceosome is activated but not catalytically primed, so that it is functionally blocked prior to the first catalytic step of splicing. The spliceosomal core is similar to the yeast B spliceosome; important differences include the presence of the RNA helicase aquarius and peptidyl prolyl isomerases. To examine the overall dynamic behavior of the purified spliceosome, we developed a principal component analysis-based approach. Calculating the energy landscape revealed eight major conformational states, which we refined to higher resolution. Conformational differences of the highly flexible structural components between these eight states reveal how spliceosomal components contribute to the assembly of the spliceosome, allowing it to generate a dynamic interaction network required for its subsequent catalytic activation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4240.map.gz emd_4240.map.gz | 245.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4240-v30.xml emd-4240-v30.xml emd-4240.xml emd-4240.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4240.png emd_4240.png | 144.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4240 http://ftp.pdbj.org/pub/emdb/structures/EMD-4240 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4240 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4240 | HTTPS FTP |
-Related structure data
| Related structure data |  6ff7MC  4233C  4234C  4235C  4236C  4237C  4238C  4239C  4247C  4248C  4249C  4250C  4251C  4252C  4253C  4254C  4255C  6ff4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10160 (Title: Conformational Dynamics of human Bact spliceosome / Data size: 2.8 TB EMPIAR-10160 (Title: Conformational Dynamics of human Bact spliceosome / Data size: 2.8 TBData #1: aligned and summed micrograph stack of human Bact spliceosome [micrographs - single frame] Data #2: aligned, dose-weighted and summed micrograph stack of human Bact spliceosome [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4240.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4240.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
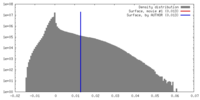
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human Bact spliceosome state 1 unmasked
| Entire | Name: human Bact spliceosome state 1 unmasked |
|---|---|
| Components |
|
-Supramolecule #1: human Bact spliceosome state 1 unmasked
| Supramolecule | Name: human Bact spliceosome state 1 unmasked / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Strain: HELA Homo sapiens (human) / Strain: HELA |
| Molecular weight | Theoretical: 4.5 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.9 Component:
| ||||||||||||
| Grid | Model: Quantifoil R3.5/1 / Material: COPPER / Mesh: 400 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 75 % / Chamber temperature: 277 K / Instrument: LEICA EM GP / Details: blot with blotting sensor. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Alignment procedure | Coma free - Residual tilt: 14.0 mrad |
| Specialist optics | Spherical aberration corrector: Microscope was modified with a Cs corrector with two hexapoles elements |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Sampling interval: 14.0 µm / Number grids imaged: 1 / Number real images: 32000 / Average exposure time: 1.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 0.001 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-6ff7: |
 Movie
Movie Controller
Controller










































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)