+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of C-terminal LRRK2 bound to MLi-2 (G2019S mutant) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GtPase / kinase / inhibitors / PROTEIN BINDING | |||||||||
| Function / homology |  Function and homology information Function and homology informationcaveola neck / negative regulation of thioredoxin peroxidase activity by peptidyl-threonine phosphorylation / negative regulation of protein processing involved in protein targeting to mitochondrion / Wnt signalosome assembly / beta-catenin destruction complex binding / regulation of branching morphogenesis of a nerve / regulation of kidney size / regulation of neuron maturation / regulation of cell projection organization / tangential migration from the subventricular zone to the olfactory bulb ...caveola neck / negative regulation of thioredoxin peroxidase activity by peptidyl-threonine phosphorylation / negative regulation of protein processing involved in protein targeting to mitochondrion / Wnt signalosome assembly / beta-catenin destruction complex binding / regulation of branching morphogenesis of a nerve / regulation of kidney size / regulation of neuron maturation / regulation of cell projection organization / tangential migration from the subventricular zone to the olfactory bulb / protein localization to endoplasmic reticulum exit site / GTP-dependent protein kinase activity / regulation of neuroblast proliferation / regulation of ER to Golgi vesicle-mediated transport / peroxidase inhibitor activity / negative regulation of late endosome to lysosome transport / regulation of mitochondrial depolarization / negative regulation of protein targeting to mitochondrion / positive regulation of dopamine receptor signaling pathway / regulation of synaptic vesicle transport / regulation of CAMKK-AMPK signaling cascade / regulation of lysosomal lumen pH / amphisome / co-receptor binding / mitochondrion localization / negative regulation of excitatory postsynaptic potential / regulation of dopamine receptor signaling pathway / regulation of retrograde transport, endosome to Golgi / positive regulation of microglial cell activation / positive regulation of synaptic vesicle endocytosis / negative regulation of autophagosome assembly / cytoplasmic side of mitochondrial outer membrane / neuron projection arborization / regulation of cAMP/PKA signal transduction / olfactory bulb development / striatum development / multivesicular body, internal vesicle / regulation of dendritic spine morphogenesis / protein localization to mitochondrion / JUN kinase kinase kinase activity / cellular response to dopamine / endoplasmic reticulum organization / positive regulation of protein autoubiquitination / Wnt signalosome / positive regulation of programmed cell death / negative regulation of protein processing / GTP metabolic process / syntaxin-1 binding / regulation of canonical Wnt signaling pathway / negative regulation of GTPase activity / exploration behavior / regulation of reactive oxygen species metabolic process / lysosome organization / Golgi-associated vesicle / clathrin binding / regulation of locomotion / phosphorylation / protein kinase A binding / negative regulation of macroautophagy / PTK6 promotes HIF1A stabilization / neuromuscular junction development / regulation of synaptic vesicle exocytosis / regulation of mitochondrial fission / Golgi organization / intracellular distribution of mitochondria / regulation of synaptic vesicle endocytosis / endoplasmic reticulum exit site / autolysosome / locomotory exploration behavior / microvillus / MAP kinase kinase kinase activity / negative regulation of Notch signaling pathway / positive regulation of protein kinase activity / Rho protein signal transduction / cellular response to manganese ion / canonical Wnt signaling pathway / presynaptic cytosol / negative regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway / regulation of synaptic transmission, glutamatergic / phagocytic vesicle / JNK cascade / positive regulation of autophagy / dendrite cytoplasm / negative regulation of protein binding / peptidyl-threonine phosphorylation / positive regulation of MAP kinase activity / tubulin binding / GTPase activator activity / SNARE binding / neuron projection morphogenesis / cellular response to starvation / positive regulation of protein ubiquitination / regulation of membrane potential / excitatory postsynaptic potential / determination of adult lifespan / cellular response to reactive oxygen species / mitochondrion organization / trans-Golgi network / calcium-mediated signaling / mitochondrial membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
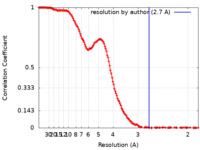
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Sanz-Murillo M / Villagran-Suarez A / Alegrio-Louro J / Leschziner A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Inhibition of Parkinson's disease-related LRRK2 by type I and type II kinase inhibitors: Activity and structures. Authors: Marta Sanz Murillo / Amalia Villagran Suarez / Verena Dederer / Deep Chatterjee / Jaime Alegrio Louro / Stefan Knapp / Sebastian Mathea / Andres E Leschziner /   Abstract: Mutations in leucine-rich repeat kinase 2 (LRRK2) are a common cause of familial Parkinson's disease (PD) and a risk factor for the sporadic form. Increased kinase activity was shown in patients with ...Mutations in leucine-rich repeat kinase 2 (LRRK2) are a common cause of familial Parkinson's disease (PD) and a risk factor for the sporadic form. Increased kinase activity was shown in patients with both familial and sporadic PD, making LRRK2 kinase inhibitors a major focus of drug development efforts. Although much progress has been made in understanding the structural biology of LRRK2, there are no available structures of LRRK2 inhibitor complexes. To this end, we solved cryo-electron microscopy structures of LRRK2, wild-type and PD-linked mutants, bound to the LRRK2-specific type I inhibitor MLi-2 and the broad-spectrum type II inhibitor GZD-824. Our structures revealed an active-like LRRK2 kinase in the type I inhibitor complex, and an inactive DYG-out in the type II inhibitor complex. Our structural analysis also showed how inhibitor-induced conformational changes in LRRK2 are affected by its autoinhibitory N-terminal repeats. The structures provide a template for the rational development of LRRK2 kinase inhibitors covering both canonical inhibitor binding modes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41754.map.gz emd_41754.map.gz | 194 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41754-v30.xml emd-41754-v30.xml emd-41754.xml emd-41754.xml | 23.3 KB 23.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41754_fsc.xml emd_41754_fsc.xml | 18.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_41754.png emd_41754.png | 81.6 KB | ||
| Filedesc metadata |  emd-41754.cif.gz emd-41754.cif.gz | 7.4 KB | ||
| Others |  emd_41754_additional_1.map.gz emd_41754_additional_1.map.gz emd_41754_half_map_1.map.gz emd_41754_half_map_1.map.gz emd_41754_half_map_2.map.gz emd_41754_half_map_2.map.gz | 7.1 MB 226.8 MB 226.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41754 http://ftp.pdbj.org/pub/emdb/structures/EMD-41754 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41754 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41754 | HTTPS FTP |
-Related structure data
| Related structure data |  8tzcMC  8txzC  8tyqC  8tzbC  8tzeC  8tzfC  8tzgC  8tzhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41754.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41754.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.935 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_41754_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_41754_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_41754_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : C-terminal LRRK2 (G2019S mutation) bound to MLi-2
| Entire | Name: C-terminal LRRK2 (G2019S mutation) bound to MLi-2 |
|---|---|
| Components |
|
-Supramolecule #1: C-terminal LRRK2 (G2019S mutation) bound to MLi-2
| Supramolecule | Name: C-terminal LRRK2 (G2019S mutation) bound to MLi-2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137 KDa |
-Macromolecule #1: Leucine-rich repeat serine/threonine-protein kinase 2
| Macromolecule | Name: Leucine-rich repeat serine/threonine-protein kinase 2 / type: protein_or_peptide / ID: 1 / Details: Several loops are missed in the structure / Number of copies: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 136.204797 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: NRMKLMIVGN TGSGKTTLLQ QLMKTKKSDL GMQSATVGID VKDWPIQIRD KRKRDLVLNV WDFAGREEFY STHPHFMTQR ALYLAVYDL SKGQAEVDAM KPWLFNIKAR ASSSPVILVG THLDVSDEKQ RKACMSKITK ELLNKRGFPA IRDYHFVNAT E ESDALAKL ...String: NRMKLMIVGN TGSGKTTLLQ QLMKTKKSDL GMQSATVGID VKDWPIQIRD KRKRDLVLNV WDFAGREEFY STHPHFMTQR ALYLAVYDL SKGQAEVDAM KPWLFNIKAR ASSSPVILVG THLDVSDEKQ RKACMSKITK ELLNKRGFPA IRDYHFVNAT E ESDALAKL RKTIINESLN FKIRDQLVVG QLIPDCYVEL EKIILSERKN VPIEFPVIDR KRLLQLVREN QLQLDENELP HA VHFLNES GVLLHFQDPA LQLSDLYFVE PKWLCKIMAQ ILTVKVEGCP KHPKGIISRR DVEKFLSKKR KFPKNYMSQY FKL LEKFQI ALPIGEEYLL VPSSLSDHRP VIELPHCENS EIIIRLYEMP YFPMGFWSRL INRLLEISPY MLSGRERALR PNRM YWRQG IYLNWSPEAY CLVGSEVLDN HPESFLKITV PSCRKGCILL GQVVDHIDSL MEEWFPGLLE IDICGEGETL LKKWA LYSF NDGEEHQKIL LDDLMKKAEE GDLLVNPDQP RLTIPISQIA PDLILADLPR NIMLNNDELE FEQAPEFLLG DGSFGS VYR AAYEGEEVAV KIFNKHTSLR LLRQELVVLC HLHHPSLISL LAAGIRPRML VMELASKGSL DRLLQQDKAS LTRTLQH RI ALHVADGLRY LHSAMIIYRD LKPHNVLLFT LYPNAAIIAK IADYSIAQYC CRMGIKTSEG TPGFRAPEVA RGNVIYNQ Q ADVYSFGLLL YDILTTGGRI VEGLKFPNEF DELEIQGKLP DPVKEYGCAP WPMVEKLIKQ CLKENPQERP TSAQVFDIL NSAELVCLTR RILLPKNVIV ECMVATHHNS RNASIWLGCG HTDRGQLSFL DLNTEGYTSE EVADSRILCL ALVHLPVEKE SWIVSGTQS GTLLVINTED GKKRHTLEKM TDSVTCLYCN SFSKQSKQKN FLLVGTADGK LAIFEDKTVK LKGAAPLKIL N IGNVSTPL MCLSESTNST ERNVMWGGCG TKIFSFSNDF TIQKLIETRT SQLFSYAAFS DSNIITVVVD TALYIAKQNS PV VEVWDKK TEKLCGLIDC VHFLREVMVK ENKESKHKMS YSGRVKTLCL QKNTALWIGT GGGHILLLDL STRRLIRVIY NFC NSVRVM MTAQLGSLKN VMLVLGYNRK NTEGTQKQKE IQSCLTVWDI NLPHEVQNLE KHIEVRKELA EKMRRTSVE UniProtKB: Leucine-rich repeat serine/threonine-protein kinase 2 |
-Macromolecule #2: E11 DARPin
| Macromolecule | Name: E11 DARPin / type: protein_or_peptide / ID: 2 Details: Some areas are missing due to the flexibility of the protein Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 19.766912 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRGSHHHHHH HHGSDLGKKL LEAARAGQDD EVRILMANGA DVNATDEAGV TPLHLAADSG HLEIVEVLLK TGADVNAWDH YGFTPLHLA AHVGHLEIVE VLLKAGADVN AQDHAGWTPL HLAALYGHLE IVEVLLKHGA DVNAQDMWGE TPFDLAIDNG N EDIAEVLQ KAAKLNDYKD DDDK |
-Macromolecule #3: (2~{R},6~{S})-2,6-dimethyl-4-[6-[5-(1-methylcyclopropyl)oxy-1~{H}...
| Macromolecule | Name: (2~{R},6~{S})-2,6-dimethyl-4-[6-[5-(1-methylcyclopropyl)oxy-1~{H}-indazol-3-yl]pyrimidin-4-yl]morpholine type: ligand / ID: 3 / Number of copies: 1 / Formula: A1N |
|---|---|
| Molecular weight | Theoretical: 379.456 Da |
| Chemical component information |  ChemComp-A1N: |
-Macromolecule #4: GUANOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 1 / Formula: GDP |
|---|---|
| Molecular weight | Theoretical: 443.201 Da |
| Chemical component information |  ChemComp-GDP: |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.822 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||||||||||||||
| Grid | Model: UltrAuFoil R2/2 / Material: GOLD / Mesh: 200 / Pretreatment - Type: PLASMA CLEANING | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: Blot force = 3 Blot time = 4 seconds Wait time = 20 seconds. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 8788 / Average exposure time: 11.0 sec. / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)