[English] 日本語
 Yorodumi
Yorodumi- EMDB-4154: Mechanism of microtubule minus-end recognition and protection by ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4154 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
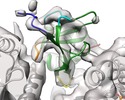
| Title | Mechanism of microtubule minus-end recognition and protection by CAMSAP proteins | |||||||||||||||||||||
 Map data Map data | CAMSAP3 CKK decorated 13pf microtubule asymmetric unit | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | CAMSAP CKK Microtubule Tubulin / Structural protein | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of organelle organization / zonula adherens maintenance / microtubule minus-end / protein transport along microtubule / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / Intraflagellar transport / Carboxyterminal post-translational modifications of tubulin / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins ...regulation of organelle organization / zonula adherens maintenance / microtubule minus-end / protein transport along microtubule / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / Intraflagellar transport / Carboxyterminal post-translational modifications of tubulin / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins / Resolution of Sister Chromatid Cohesion / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / microtubule anchoring / COPI-dependent Golgi-to-ER retrograde traffic / regulation of Golgi organization / COPI-independent Golgi-to-ER retrograde traffic / COPI-mediated anterograde transport / RHO GTPases activate IQGAPs / microtubule minus-end binding / RHO GTPases Activate Formins / establishment or maintenance of microtubule cytoskeleton polarity / MHC class II antigen presentation / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / cilium movement / Aggrephagy / epithelial cell-cell adhesion / The role of GTSE1 in G2/M progression after G2 checkpoint / Separation of Sister Chromatids / zonula adherens / Loss of Nlp from mitotic centrosomes / Recruitment of mitotic centrosome proteins and complexes / Loss of proteins required for interphase microtubule organization from the centrosome / Anchoring of the basal body to the plasma membrane / AURKA Activation by TPX2 / Recruitment of NuMA to mitotic centrosomes / Regulation of PLK1 Activity at G2/M Transition / Hedgehog 'off' state / establishment of epithelial cell apical/basal polarity / positive regulation of axon guidance / embryo development ending in birth or egg hatching / motile cilium / regulation of focal adhesion assembly / spectrin binding / regulation of microtubule polymerization / axoneme / microtubule-based process / cytoplasmic microtubule / regulation of microtubule cytoskeleton organization / cellular response to interleukin-4 / regulation of cell migration / structural constituent of cytoskeleton / microtubule cytoskeleton organization / neuron migration / neuron projection development / actin filament binding / mitotic cell cycle / double-stranded RNA binding / microtubule cytoskeleton / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / in utero embryonic development / microtubule / hydrolase activity / calmodulin binding / ciliary basal body / cilium / protein heterodimerization activity / GTPase activity / centrosome / ubiquitin protein ligase binding / GTP binding / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||||||||||||||
| Biological species |   | |||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.3 Å | |||||||||||||||||||||
 Authors Authors | Akhmanova A / Moores CA | |||||||||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  United States, United States,  Netherlands, Netherlands,  Switzerland, 6 items Switzerland, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2017 Journal: Nat Struct Mol Biol / Year: 2017Title: A structural model for microtubule minus-end recognition and protection by CAMSAP proteins. Authors: Joseph Atherton / Kai Jiang / Marcel M Stangier / Yanzhang Luo / Shasha Hua / Klaartje Houben / Jolien J E van Hooff / Agnel-Praveen Joseph / Guido Scarabelli / Barry J Grant / Anthony J ...Authors: Joseph Atherton / Kai Jiang / Marcel M Stangier / Yanzhang Luo / Shasha Hua / Klaartje Houben / Jolien J E van Hooff / Agnel-Praveen Joseph / Guido Scarabelli / Barry J Grant / Anthony J Roberts / Maya Topf / Michel O Steinmetz / Marc Baldus / Carolyn A Moores / Anna Akhmanova /     Abstract: CAMSAP and Patronin family members regulate microtubule minus-end stability and localization and thus organize noncentrosomal microtubule networks, which are essential for cell division, polarization ...CAMSAP and Patronin family members regulate microtubule minus-end stability and localization and thus organize noncentrosomal microtubule networks, which are essential for cell division, polarization and differentiation. Here, we found that the CAMSAP C-terminal CKK domain is widely present among eukaryotes and autonomously recognizes microtubule minus ends. Through a combination of structural approaches, we uncovered how mammalian CKK binds between two tubulin dimers at the interprotofilament interface on the outer microtubule surface. In vitro reconstitution assays combined with high-resolution fluorescence microscopy and cryo-electron tomography suggested that CKK preferentially associates with the transition zone between curved protofilaments and the regular microtubule lattice. We propose that minus-end-specific features of the interprotofilament interface at this site serve as the basis for CKK's minus-end preference. The steric clash between microtubule-bound CKK and kinesin motors explains how CKK protects microtubule minus ends against kinesin-13-induced depolymerization and thus controls the stability of free microtubule minus ends. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4154.map.gz emd_4154.map.gz | 715.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4154-v30.xml emd-4154-v30.xml emd-4154.xml emd-4154.xml | 17.4 KB 17.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4154.png emd_4154.png | 269.5 KB | ||
| Filedesc metadata |  emd-4154.cif.gz emd-4154.cif.gz | 6.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4154 http://ftp.pdbj.org/pub/emdb/structures/EMD-4154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4154 | HTTPS FTP |
-Related structure data
| Related structure data |  5m50MC  3444C  4156C  5lznC  5m54C  5m5cC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4154.map.gz / Format: CCP4 / Size: 2.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4154.map.gz / Format: CCP4 / Size: 2.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CAMSAP3 CKK decorated 13pf microtubule asymmetric unit | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.534 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Complex of two tubulin dimers with bound CAMSAP3-CKK domain
+Supramolecule #1: Complex of two tubulin dimers with bound CAMSAP3-CKK domain
+Supramolecule #2: tubulin
+Supramolecule #3: CAMSAP3-CKK domain
+Macromolecule #1: Tubulin alpha chain
+Macromolecule #2: Tubulin beta-2B chain
+Macromolecule #3: Calmodulin-regulated spectrin-associated protein 3
+Macromolecule #4: GUANOSINE-5'-TRIPHOSPHATE
+Macromolecule #5: MAGNESIUM ION
+Macromolecule #6: GUANOSINE-5'-DIPHOSPHATE
+Macromolecule #7: TAXOL
+Macromolecule #8: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 6.8 / Details: BRB80 |
|---|---|
| Grid | Model: C-flat-2/2 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK III |
| Details | 13pf Microtubules |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: DIRECT ELECTRON DE-20 (5k x 3k) / Detector mode: INTEGRATING / Average electron dose: 25.0 e/Å2 / Details: 25e-/A2 used in final reconstuctions |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: Kinesin decorated microtubule |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 5.3 Å / Resolution method: FSC 0.143 CUT-OFF Details: Gold-standard noise substitution test used to asses for over fitting (Chen et al., 2013) Number images used: 6530 |
| Initial angle assignment | Type: PROJECTION MATCHING / Software - Name: SPIDER |
| Final angle assignment | Type: OTHER / Software - Name: FREALIGN / Details: Projection matching in Fourier space |
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|---|
| Output model |  PDB-5m50: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)


























