[English] 日本語
 Yorodumi
Yorodumi- EMDB-39192: Cryo-EM structure of in vitro reconstituted Light-harvesting comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of in vitro reconstituted Light-harvesting complex II trimer | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Complex / light-harvesting / membrane protein / PHOTOSYNTHESIS | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationphotosynthesis, light harvesting in photosystem I / photosystem I / photosystem II / chlorophyll binding / chloroplast thylakoid membrane / response to light stimulus / metal ion binding Similarity search - Function | ||||||||||||||||||
| Biological species |  Spinacia oleracea (spinach) Spinacia oleracea (spinach) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.37 Å | ||||||||||||||||||
 Authors Authors | Seki S / Miyata T / Tanaka H / Namba K / Kurisu G / Fujii R | ||||||||||||||||||
| Funding support |  Japan, 5 items Japan, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: PNAS Nexus / Year: 2024 Journal: PNAS Nexus / Year: 2024Title: Structure-based validation of recombinant light-harvesting complex II. Authors: Soichiro Seki / Tomoko Miyata / Naoko Norioka / Hideaki Tanaka / Genji Kurisu / Keiichi Namba / Ritsuko Fujii /  Abstract: Light-harvesting complex II (LHCII) captures sunlight and dissipates excess energy to drive photosynthesis. To elucidate this mechanism, the individual optical properties of pigments in the LHCII ...Light-harvesting complex II (LHCII) captures sunlight and dissipates excess energy to drive photosynthesis. To elucidate this mechanism, the individual optical properties of pigments in the LHCII protein must be identified. In vitro reconstitution with apoproteins synthesized by and pigment-lipid mixtures from natural sources is an effective approach; however, the local environment surrounding each pigment within reconstituted LHCII (rLHCII) has only been indirectly estimated using spectroscopic and biochemical methods. Here, we used cryo-electron microscopy to determine the 3D structure of the rLHCII trimer and found that rLHCII exhibited a structure that was virtually identical to that of native LHCII, with a few exceptions: some C-terminal amino acids were not visible, likely due to aggregation of the His-tags; a carotenoid at the V1 site was not visible; and at site 614 showed mixed occupancy by both chlorophyll and molecules. Our observations confirmed the applicability of the in vitro reconstitution technique. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39192.map.gz emd_39192.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39192-v30.xml emd-39192-v30.xml emd-39192.xml emd-39192.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39192_fsc.xml emd_39192_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_39192.png emd_39192.png | 34.4 KB | ||
| Filedesc metadata |  emd-39192.cif.gz emd-39192.cif.gz | 6.7 KB | ||
| Others |  emd_39192_half_map_1.map.gz emd_39192_half_map_1.map.gz emd_39192_half_map_2.map.gz emd_39192_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39192 http://ftp.pdbj.org/pub/emdb/structures/EMD-39192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39192 | HTTPS FTP |
-Related structure data
| Related structure data |  8yeeMC  8y15C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39192.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39192.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.873 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_39192_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_39192_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : In vitro reconstituted Light-harvesting complex II
| Entire | Name: In vitro reconstituted Light-harvesting complex II |
|---|---|
| Components |
|
-Supramolecule #1: In vitro reconstituted Light-harvesting complex II
| Supramolecule | Name: In vitro reconstituted Light-harvesting complex II / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 120 KDa |
-Macromolecule #1: Chlorophyll a-b binding protein, chloroplastic
| Macromolecule | Name: Chlorophyll a-b binding protein, chloroplastic / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 24.455611 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SPWYGPDRVK YLGPFSGESP SYLTGEFPGD YGWDTAGLSA DPETFAKNRE LEVIHCRWAM LGALGCVFPE LLARNGVKFG EAVWFKAGS QIFSEGGLDY LGNPSLVHAQ SILAIWACQV ILMGAVEGYR IAGGPLGEVV DPLYPGGSFD PLGLADDPEA F AELKVKEI ...String: SPWYGPDRVK YLGPFSGESP SYLTGEFPGD YGWDTAGLSA DPETFAKNRE LEVIHCRWAM LGALGCVFPE LLARNGVKFG EAVWFKAGS QIFSEGGLDY LGNPSLVHAQ SILAIWACQV ILMGAVEGYR IAGGPLGEVV DPLYPGGSFD PLGLADDPEA F AELKVKEI KNGRLAMFSM FGFFVQAIVT GKGPLENLAD HLADPVNNNA WNFATNFVPG KHHHHHH UniProtKB: Chlorophyll a-b binding protein, chloroplastic |
-Macromolecule #2: CHLOROPHYLL B
| Macromolecule | Name: CHLOROPHYLL B / type: ligand / ID: 2 / Number of copies: 18 / Formula: CHL |
|---|---|
| Molecular weight | Theoretical: 907.472 Da |
| Chemical component information |  ChemComp-CHL: |
-Macromolecule #3: CHLOROPHYLL A
| Macromolecule | Name: CHLOROPHYLL A / type: ligand / ID: 3 / Number of copies: 24 / Formula: CLA |
|---|---|
| Molecular weight | Theoretical: 893.489 Da |
| Chemical component information |  ChemComp-CLA: |
-Macromolecule #4: Lutein
| Macromolecule | Name: Lutein / type: ligand / ID: 4 / Number of copies: 6 / Formula: A1LXP |
|---|---|
| Molecular weight | Theoretical: 568.871 Da |
-Macromolecule #5: (1R,3R)-6-{(3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4R,6R)-4-HYDROXY...
| Macromolecule | Name: (1R,3R)-6-{(3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4R,6R)-4-HYDROXY-2,2,6-TRIMETHYL-7-OXABICYCLO[4.1.0]HEPT-1-YL]-3,7,12,16-TETRAMETHYLOCTADECA-1,3,5,7,9,11,13,15,17-NONAENYLIDENE}-1,5,5-TRIMETHYLCYCLOHEXANE-1,3-DIOL type: ligand / ID: 5 / Number of copies: 3 / Formula: NEX |
|---|---|
| Molecular weight | Theoretical: 600.87 Da |
| Chemical component information | 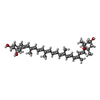 ChemComp-NEX: |
-Macromolecule #6: 1,2-DIPALMITOYL-PHOSPHATIDYL-GLYCEROLE
| Macromolecule | Name: 1,2-DIPALMITOYL-PHOSPHATIDYL-GLYCEROLE / type: ligand / ID: 6 / Number of copies: 3 / Formula: LHG |
|---|---|
| Molecular weight | Theoretical: 722.97 Da |
| Chemical component information |  ChemComp-LHG: |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 126 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Component - Concentration: 25.0 mM / Component - Formula: C8H18N2O4S / Component - Name: HEPES / Details: 0.03 % n-Dodexyl-alpha-D-maltoside |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 10 sec. / Pretreatment - Pressure: 10.0 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 6145 / Average exposure time: 3.0 sec. / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 60000 |
| Sample stage | Specimen holder model: JEOL CRYOSPECPORTER / Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






































