[English] 日本語
 Yorodumi
Yorodumi- EMDB-3718: 3,4-dihydroxybenzoate decarboxylase AroY from Enterobacter cloaca... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3718 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 3,4-dihydroxybenzoate decarboxylase AroY from Enterobacter cloacae in the apo state | |||||||||
 Map data Map data | Single-particle cryo-EM map of the 3,4-dihydroxybenzoate decarboxylase AroY from Enterobacter cloacae in the apo state. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Enterobacter cloacae (bacteria) Enterobacter cloacae (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.6 Å | |||||||||
 Authors Authors | Baerland N / Kaltwasser S / Vonck J / Pavkov-Keller T | |||||||||
 Citation Citation |  Journal: Angew Chem Int Ed Engl / Year: 2017 Journal: Angew Chem Int Ed Engl / Year: 2017Title: Regioselective para-Carboxylation of Catechols with a Prenylated Flavin Dependent Decarboxylase. Authors: Stefan E Payer / Stephen A Marshall / Natalie Bärland / Xiang Sheng / Tamara Reiter / Andela Dordic / Georg Steinkellner / Christiane Wuensch / Susann Kaltwasser / Karl Fisher / Stephen E J ...Authors: Stefan E Payer / Stephen A Marshall / Natalie Bärland / Xiang Sheng / Tamara Reiter / Andela Dordic / Georg Steinkellner / Christiane Wuensch / Susann Kaltwasser / Karl Fisher / Stephen E J Rigby / Peter Macheroux / Janet Vonck / Karl Gruber / Kurt Faber / Fahmi Himo / David Leys / Tea Pavkov-Keller / Silvia M Glueck /     Abstract: The utilization of CO as a carbon source for organic synthesis meets the urgent demand for more sustainability in the production of chemicals. Herein, we report on the enzyme-catalyzed para- ...The utilization of CO as a carbon source for organic synthesis meets the urgent demand for more sustainability in the production of chemicals. Herein, we report on the enzyme-catalyzed para-carboxylation of catechols, employing 3,4-dihydroxybenzoic acid decarboxylases (AroY) that belong to the UbiD enzyme family. Crystal structures and accompanying solution data confirmed that AroY utilizes the recently discovered prenylated FMN (prFMN) cofactor, and requires oxidative maturation to form the catalytically competent prFMN species. This study reports on the in vitro reconstitution and activation of a prFMN-dependent enzyme that is capable of directly carboxylating aromatic catechol substrates under ambient conditions. A reaction mechanism for the reversible decarboxylation involving an intermediate with a single covalent bond between a quinoid adduct and cofactor is proposed, which is distinct from the mechanism of prFMN-associated 1,3-dipolar cycloadditions in related enzymes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3718.map.gz emd_3718.map.gz | 39.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3718-v30.xml emd-3718-v30.xml emd-3718.xml emd-3718.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3718_fsc.xml emd_3718_fsc.xml | 7.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_3718.png emd_3718.png | 203.9 KB | ||
| Others |  emd_3718_half_map_1.map.gz emd_3718_half_map_1.map.gz emd_3718_half_map_2.map.gz emd_3718_half_map_2.map.gz | 33 MB 33 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3718 http://ftp.pdbj.org/pub/emdb/structures/EMD-3718 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3718 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3718 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3718.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3718.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Single-particle cryo-EM map of the 3,4-dihydroxybenzoate decarboxylase AroY from Enterobacter cloacae in the apo state. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.12 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
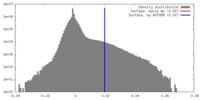
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: half-map2 of 3,4-dihydroxybenzoate decarboxylase AroY
| File | emd_3718_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map2 of 3,4-dihydroxybenzoate decarboxylase AroY | ||||||||||||
| Projections & Slices |
| ||||||||||||
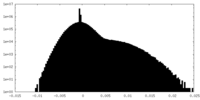
| Density Histograms |
-Half map: half-map1 of 3,4-dihydroxybenzoate decarboxylase AroY
| File | emd_3718_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map1 of 3,4-dihydroxybenzoate decarboxylase AroY | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : 3,4-dihydroxybenzoate decarboxylase
| Entire | Name: 3,4-dihydroxybenzoate decarboxylase |
|---|---|
| Components |
|
-Supramolecule #1: 3,4-dihydroxybenzoate decarboxylase
| Supramolecule | Name: 3,4-dihydroxybenzoate decarboxylase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Enterobacter cloacae (bacteria) Enterobacter cloacae (bacteria) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 320 KDa |
-Macromolecule #1: 3,4-dihydroxybenzoate decarboxylase
| Macromolecule | Name: 3,4-dihydroxybenzoate decarboxylase / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MQNPINDLRS AIALLQRHPG HYIETDHPVD PNAELAGVYR HIGAGGTVKR PTRTGPAMMF NSVKGYPGS RILVGMHASR ERAALLLGCV PSKLAQHVGQ AVKNPVAPVV VPASQAPCQE Q VFYADDPD FDLRKLLPAP TNTPIDAGPF FCLGLVLASD PEDTSLTDVT ...String: MQNPINDLRS AIALLQRHPG HYIETDHPVD PNAELAGVYR HIGAGGTVKR PTRTGPAMMF NSVKGYPGS RILVGMHASR ERAALLLGCV PSKLAQHVGQ AVKNPVAPVV VPASQAPCQE Q VFYADDPD FDLRKLLPAP TNTPIDAGPF FCLGLVLASD PEDTSLTDVT IHRLCVQERD EL SMFLAAG RHIEVFRKKA EAAGKPLPVT INMGLDPAIY IGACFEAPTT PFGYNELGVA GAL RQQPVE LVQGVAVKEK AIARAEIIIE GELLPGVRVR EDQHTNTGHA MPEFPGYCGE ANPS LPVIK VKAVTMRNHA ILQTLVGPGE EHTTLAGLPT EASIRNAVEE AIPGFLQNVY AHTAG GGKF LGILQVKKRQ PSDEGRQGQA ALIALATYSE LKNIILVDED VDIFDSDDIL WAMTTR MQG DVSITTLPGI RGHQLDPSQS PDYSTSIRGN GISCKTIFDC TVPWALKARF ERAPFME VD PTPWAPELFS DKK |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV / Details: 11 second blot. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3200FSC |
|---|---|
| Specialist optics | Energy filter - Name: JEOL in-column filter with a width of 20eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 8.0 sec. / Average electron dose: 54.0 e/Å2 / Details: 40-frame movies in 8 seconds |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 3.0 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 44643 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 4.2 mm / Nominal magnification: 30000 |
| Sample stage | Specimen holder model: JEOL 3200FSC CRYOHOLDER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | The crystal structure of the same molecule was rigid-body fitted in the map. |
|---|---|
| Refinement | Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)