+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM map of LonC protease open hexamer with Bortezomib | |||||||||
 Map data Map data | CryoEM of map of LonC open hexamer with Bortezomib | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Lon proteases / chaperone / open hexamer / inhibitor | |||||||||
| Biological species |  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.5 Å | |||||||||
 Authors Authors | Li M / Hsieh K / Liu H / Zhang S / Gao Y / Gong Q / Zhang K / Chang C / Li S | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Fundam Res / Year: 2024 Journal: Fundam Res / Year: 2024Title: Bifurcated assembly pathway and dual function of a Lon-like protease revealed by cryo-EM Analysis Authors: Li M / Liu H / Hsieh KY / Zhang S / Gao Y / Gong Q / Zhang K / Chang CI / Li S | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36978.map.gz emd_36978.map.gz | 136.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36978-v30.xml emd-36978-v30.xml emd-36978.xml emd-36978.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36978.png emd_36978.png | 25.7 KB | ||
| Filedesc metadata |  emd-36978.cif.gz emd-36978.cif.gz | 5 KB | ||
| Others |  emd_36978_additional_1.map.gz emd_36978_additional_1.map.gz emd_36978_half_map_1.map.gz emd_36978_half_map_1.map.gz emd_36978_half_map_2.map.gz emd_36978_half_map_2.map.gz | 71.2 MB 134.5 MB 134.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36978 http://ftp.pdbj.org/pub/emdb/structures/EMD-36978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36978 | HTTPS FTP |
-Validation report
| Summary document |  emd_36978_validation.pdf.gz emd_36978_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36978_full_validation.pdf.gz emd_36978_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_36978_validation.xml.gz emd_36978_validation.xml.gz | 14.4 KB | Display | |
| Data in CIF |  emd_36978_validation.cif.gz emd_36978_validation.cif.gz | 17 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36978 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36978 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36978 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36978 | HTTPS FTP |
-Related structure data
| Related structure data |  8k8vC  8k8wC  8k8xC  8k8yC  8k8zC  8k90C  8k91C  8k92C  8k93C  8k94C  8k95C  8k96C  8k97C C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36978.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36978.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM of map of LonC open hexamer with Bortezomib | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
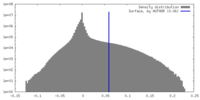
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: CryoEM of map of LonC open hexamer with Bortezomib
| File | emd_36978_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM of map of LonC open hexamer with Bortezomib | ||||||||||||
| Projections & Slices |
| ||||||||||||
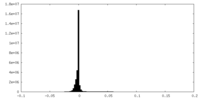
| Density Histograms |
-Half map: CryoEM of map of LonC open hexamer with Bortezomib
| File | emd_36978_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM of map of LonC open hexamer with Bortezomib | ||||||||||||
| Projections & Slices |
| ||||||||||||
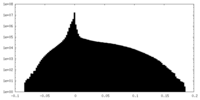
| Density Histograms |
-Half map: CryoEM of map of LonC open hexamer with Bortezomib
| File | emd_36978_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM of map of LonC open hexamer with Bortezomib | ||||||||||||
| Projections & Slices |
| ||||||||||||
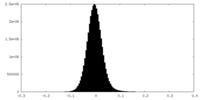
| Density Histograms |
- Sample components
Sample components
-Entire : CryoEM structure of LonC protease open hexamer with Bortezomib
| Entire | Name: CryoEM structure of LonC protease open hexamer with Bortezomib |
|---|---|
| Components |
|
-Supramolecule #1: CryoEM structure of LonC protease open hexamer with Bortezomib
| Supramolecule | Name: CryoEM structure of LonC protease open hexamer with Bortezomib type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Molecular weight | Theoretical: 480 KDa |
-Macromolecule #1: LonC open hexamer with Bortezomib
| Macromolecule | Name: LonC open hexamer with Bortezomib / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Recombinant expression | Organism:  |
| Sequence | String: MRLSYEALEW RTPIENSTEP VSLPPPPPFF GQERAREALE LAIRGGFHAY LVGPPSLGKH EALLAYLSTQ SVETPPDLLY VPLSERKVAV LTLPSGQEIH LAEAVEGLLL EVNRLDELFR QGSFLREKTQ LEARFKEARE QQLEALRREA QEAGFALSTN GERLELTGPG ...String: MRLSYEALEW RTPIENSTEP VSLPPPPPFF GQERAREALE LAIRGGFHAY LVGPPSLGKH EALLAYLSTQ SVETPPDLLY VPLSERKVAV LTLPSGQEIH LAEAVEGLLL EVNRLDELFR QGSFLREKTQ LEARFKEARE QQLEALRREA QEAGFALSTN GERLELTGPG PVPAELSARL EEVTLGSLAA SAELEVALRR LRRDWALHYL NNRFEPLFQR FPQARAYLEA LRARLARYAE TGEPLDPAQW RPNLLTSSSS GTPPPIVYEP YATAPRLFGR LDYLVDRGVW STNVSLIRPG AVHRAQGGYL ILDALSLKRE GTWEAFKRAL RNGQVEPVTE PQAPAGLEVE PFPIQMQVIL VGTPEAFEGL EEDPAFSELF RIRAEFSPTL PASPENCTAL GGWLLAQGFQ LTQGGLTRLY DEARRMAEQR DRMDARLVEI RALAEEAAVL GGGLLTAESV EQAIAAREHR SFLSEEEFLR AVQEGVIRLR TTGRAVGEVN SLVVVEAAPY WGRPARLTAR AAPGRDHLIS IDREAGLGGQ IFHKAVLTLA GYLRSRYIEH GSLPVTISLA FEQNYVSIEG DSAGLAELVA ALSAIGNLPL RQDLAVTGAV DQTGKVLAVG AINAKVEGFF RVCKALGLSG TQGVILPEAN LANLTLRAEV LEAVRAGQFH IYAVETAEQA LEILAGARME GFRGLQEKIR AGLEAFARLE EGHDKEDREK LAAALEHHHH HH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.7 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)