[English] 日本語
 Yorodumi
Yorodumi- EMDB-3524: cryoEM Structure of Polycystin-2 in complex with calcium and lipids -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3524 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryoEM Structure of Polycystin-2 in complex with calcium and lipids | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ca2+ signaling / cryoEM / membrane protein structure / Polycystin-2 / TRP channel / transport protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationdetection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development / polycystin complex / mesonephric tubule development / mesonephric duct development / metanephric part of ureteric bud development / renal tubule morphogenesis ...detection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development / polycystin complex / mesonephric tubule development / mesonephric duct development / metanephric part of ureteric bud development / renal tubule morphogenesis / determination of liver left/right asymmetry / metanephric ascending thin limb development / metanephric mesenchyme development / metanephric S-shaped body morphogenesis / basal cortex / renal artery morphogenesis / HLH domain binding / calcium-induced calcium release activity / migrasome / cilium organization / VxPx cargo-targeting to cilium / detection of mechanical stimulus / muscle alpha-actinin binding / regulation of calcium ion import / voltage-gated monoatomic ion channel activity / placenta blood vessel development / cellular response to hydrostatic pressure / cellular response to fluid shear stress / cation channel complex / outward rectifier potassium channel activity / non-motile cilium / actinin binding / cellular response to osmotic stress / determination of left/right symmetry / : / voltage-gated monoatomic cation channel activity / neural tube development / voltage-gated sodium channel activity / aorta development / motile cilium / ciliary membrane / branching involved in ureteric bud morphogenesis / protein heterotetramerization / negative regulation of G1/S transition of mitotic cell cycle / spinal cord development / heart looping / positive regulation of phospholipase C-activating G protein-coupled receptor signaling pathway / cytoplasmic side of endoplasmic reticulum membrane / centrosome duplication / voltage-gated potassium channel activity / cell surface receptor signaling pathway via JAK-STAT / potassium channel activity / embryonic placenta development / monoatomic cation channel activity / voltage-gated calcium channel activity / transcription regulator inhibitor activity / cytoskeletal protein binding / release of sequestered calcium ion into cytosol / potassium ion transmembrane transport / sodium ion transmembrane transport / cellular response to calcium ion / basal plasma membrane / cytoplasmic vesicle membrane / cellular response to cAMP / lumenal side of endoplasmic reticulum membrane / cellular response to reactive oxygen species / protein tetramerization / phosphoprotein binding / establishment of localization in cell / liver development / calcium ion transmembrane transport / Wnt signaling pathway / intracellular calcium ion homeostasis / positive regulation of nitric oxide biosynthetic process / mitotic spindle / cell-cell junction / calcium ion transport / lamellipodium / regulation of cell population proliferation / heart development / ATPase binding / protein homotetramerization / basolateral plasma membrane / transmembrane transporter binding / cell surface receptor signaling pathway / regulation of cell cycle / cilium / ciliary basal body / signaling receptor binding / negative regulation of cell population proliferation / calcium ion binding / positive regulation of gene expression / endoplasmic reticulum membrane / endoplasmic reticulum / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Wilkes M / Madej MG | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2017 Journal: Nat Struct Mol Biol / Year: 2017Title: Molecular insights into lipid-assisted Ca regulation of the TRP channel Polycystin-2. Authors: Martin Wilkes / M Gregor Madej / Lydia Kreuter / Daniel Rhinow / Veronika Heinz / Silvia De Sanctis / Sabine Ruppel / Rebecca M Richter / Friederike Joos / Marina Grieben / Ashley C W Pike / ...Authors: Martin Wilkes / M Gregor Madej / Lydia Kreuter / Daniel Rhinow / Veronika Heinz / Silvia De Sanctis / Sabine Ruppel / Rebecca M Richter / Friederike Joos / Marina Grieben / Ashley C W Pike / Juha T Huiskonen / Elisabeth P Carpenter / Werner Kühlbrandt / Ralph Witzgall / Christine Ziegler /   Abstract: Polycystin-2 (PC2), a calcium-activated cation TRP channel, is involved in diverse Ca signaling pathways. Malfunctioning Ca regulation in PC2 causes autosomal-dominant polycystic kidney disease. Here ...Polycystin-2 (PC2), a calcium-activated cation TRP channel, is involved in diverse Ca signaling pathways. Malfunctioning Ca regulation in PC2 causes autosomal-dominant polycystic kidney disease. Here we report two cryo-EM structures of distinct channel states of full-length human PC2 in complex with lipids and cations. The structures reveal conformational differences in the selectivity filter and in the large exoplasmic domain (TOP domain), which displays differing N-glycosylation. The more open structure has one cation bound below the selectivity filter (single-ion mode, PC2), whereas multiple cations are bound along the translocation pathway in the second structure (multi-ion mode, PC2). Ca binding at the entrance of the selectivity filter suggests Ca blockage in PC2, and we observed density for the Ca-sensing C-terminal EF hand in the unblocked PC2 state. The states show altered interactions of lipids with the pore loop and TOP domain, thus reflecting the functional diversity of PC2 at different locations, owing to different membrane compositions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3524.map.gz emd_3524.map.gz | 4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3524-v30.xml emd-3524-v30.xml emd-3524.xml emd-3524.xml | 21.3 KB 21.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3524_fsc.xml emd_3524_fsc.xml | 7 KB | Display |  FSC data file FSC data file |
| Images |  emd_3524.png emd_3524.png | 98.1 KB | ||
| Filedesc metadata |  emd-3524.cif.gz emd-3524.cif.gz | 7.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3524 http://ftp.pdbj.org/pub/emdb/structures/EMD-3524 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3524 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3524 | HTTPS FTP |
-Validation report
| Summary document |  emd_3524_validation.pdf.gz emd_3524_validation.pdf.gz | 468.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3524_full_validation.pdf.gz emd_3524_full_validation.pdf.gz | 468.5 KB | Display | |
| Data in XML |  emd_3524_validation.xml.gz emd_3524_validation.xml.gz | 9 KB | Display | |
| Data in CIF |  emd_3524_validation.cif.gz emd_3524_validation.cif.gz | 11.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3524 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3524 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3524 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3524 | HTTPS FTP |
-Related structure data
| Related structure data |  5mkfMC  3523C  5mkeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3524.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3524.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.14 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Polycystin-2
| Entire | Name: Polycystin-2 |
|---|---|
| Components |
|
-Supramolecule #1: Polycystin-2
| Supramolecule | Name: Polycystin-2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Polycystin-2
| Macromolecule | Name: Polycystin-2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 109.820086 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MVNSSRVQPQ QPGDAKRPPA PRAPDPGRLM AGCAAVGASL AAPGGLCEQR GLEIEMQRIR QAAARDPPAG AAASPSPPLS SCSRQAWSR DNPGFEAEEE EEEVEGEEGG MVVEMDVEWR PGSRRSAASS AVSSVGARSR GLGGYHGAGH PSGRRRRRED Q GPPCPSPV ...String: MVNSSRVQPQ QPGDAKRPPA PRAPDPGRLM AGCAAVGASL AAPGGLCEQR GLEIEMQRIR QAAARDPPAG AAASPSPPLS SCSRQAWSR DNPGFEAEEE EEEVEGEEGG MVVEMDVEWR PGSRRSAASS AVSSVGARSR GLGGYHGAGH PSGRRRRRED Q GPPCPSPV GGGDPLHRHL PLEGQPPRVA WAERLVRGLR GLWGTRLMEE SSTNREKYLK SVLRELVTYL LFLIVLCILT YG MMSSNVY YYTRMMSQLF LDTPVSKTEK TNFKTLSSME DFWKFTEGSL LDGLYWKMQP SNQTEADNRS FIFYENLLLG VPR IRQLRV RNGSCSIPQD LRDEIKECYD VYSVSSEDRA PFGPRNGTAW IYTSEKDLNG SSHWGIIATY SGAGYYLDLS RTRE ETAAQ VASLKKNVWL DRGTRATFID FSVYNANINL FCVVRLLVEF PATGGVIPSW QFQPLKLIRY VTTFDFFLAA CEIIF CFFI FYYVVEEILE IRIHKLHYFR SFWNCLDVVI VVLSVVAIGI NIYRTSNVEV LLQFLEDQNT FPNFEHLAYW QIQFNN IAA VTVFFVWIKL FKFINFNRTM SQLSTTMSRC AKDLFGFAIM FFIIFLAYAQ LAYLVFGTQV DDFSTFQECI FTQFRII LG DINFAEIEEA NRVLGPIYFT TFVFFMFFIL LNMFLAIIND TYSEVKSDLA QQKAEMELSD LIRKGYHKAL VKLKLKKN T VDDISESLRQ GGGKLNFDEL RQDLKGKGHT DAEIEAIFTK YDQDGDQELT EHEHQQMRDD LEKEREDLDL DHSSLPRPM SSRSFPRSLD DSEEDDDEDS GHSSRRRGSI SSGVSYEEFQ VLVRRVDRME HSIGSIVSKI DAVIVKLEIM ERAKLKRREV LGRLLDGVA EDERLGRDSE IHREQMERLV REELERWESD DAASQISHGL GTPVGLNGQP RPRSSRPSSS QSTEGMEGAG G NGSSNVHV UniProtKB: Polycystin-2 |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 12 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHATE
| Macromolecule | Name: 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHATE / type: ligand / ID: 4 / Number of copies: 4 / Formula: PX6 |
|---|---|
| Molecular weight | Theoretical: 647.883 Da |
| Chemical component information |  ChemComp-PX6: |
-Macromolecule #5: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 5 / Number of copies: 12 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Macromolecule #6: 4-AMINO-5-CYCLOHEXYL-3-HYDROXY-PENTANOIC ACID
| Macromolecule | Name: 4-AMINO-5-CYCLOHEXYL-3-HYDROXY-PENTANOIC ACID / type: ligand / ID: 6 / Number of copies: 8 / Formula: CHS |
|---|---|
| Molecular weight | Theoretical: 215.289 Da |
| Chemical component information | 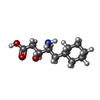 ChemComp-CHS: |
-Macromolecule #7: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 7 / Number of copies: 5 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3200FSC |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 1.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 4.1 mm |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | we used for comparative structure modeling TRPA1 (pdb entry code 3J9P) as template for S1 and S3-S5, TRPV1 (pdb entry code 3J5Q) for S5-S6, and the TRPV2 (pdb entry code 5AN8) fitted best for S2-S3 to obtain an initial model. The soluble domain was build based on pdbID: 5K47. But we had no search model for molecular replacement. Although we had a good idea what the architecture would be like, we build the model de novo with COOT. |
|---|---|
| Refinement | Space: REAL |
| Output model |  PDB-5mkf: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)























