+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31541 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
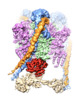
| Title | CryoEM Structures of Reconstituted V-ATPase, Oxr1 bound V1 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ATPase / proton pump / rotary motor enzyme / membrane protein / MOTOR PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationvacuole-mitochondrion membrane contact site / protein retention in Golgi apparatus / Insulin receptor recycling / Transferrin endocytosis and recycling / ROS and RNS production in phagocytes / Amino acids regulate mTORC1 / Golgi lumen acidification / proteasome storage granule assembly / protein-containing complex disassembly / vacuolar proton-transporting V-type ATPase, V1 domain ...vacuole-mitochondrion membrane contact site / protein retention in Golgi apparatus / Insulin receptor recycling / Transferrin endocytosis and recycling / ROS and RNS production in phagocytes / Amino acids regulate mTORC1 / Golgi lumen acidification / proteasome storage granule assembly / protein-containing complex disassembly / vacuolar proton-transporting V-type ATPase, V1 domain / endosomal lumen acidification / proton-transporting V-type ATPase complex / pexophagy / vacuolar proton-transporting V-type ATPase complex / vacuolar acidification / fungal-type vacuole membrane / proton-transporting ATPase activity, rotational mechanism / ATP metabolic process / Neutrophil degranulation / proton transmembrane transport / transmembrane transport / cytoplasmic stress granule / intracellular calcium ion homeostasis / response to oxidative stress / membrane raft / Golgi membrane / mitochondrion / ATP binding / membrane / nucleus / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |   | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||||||||
 Authors Authors | Khan MM / Lee S | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: EMBO J / Year: 2022 Journal: EMBO J / Year: 2022Title: Oxidative stress protein Oxr1 promotes V-ATPase holoenzyme disassembly in catalytic activity-independent manner. Authors: Md Murad Khan / Seowon Lee / Sergio Couoh-Cardel / Rebecca A Oot / Hyunmin Kim / Stephan Wilkens / Soung-Hun Roh /   Abstract: The vacuolar ATPase (V-ATPase) is a rotary motor proton pump that is regulated by an assembly equilibrium between active holoenzyme and autoinhibited V -ATPase and V proton channel subcomplexes. ...The vacuolar ATPase (V-ATPase) is a rotary motor proton pump that is regulated by an assembly equilibrium between active holoenzyme and autoinhibited V -ATPase and V proton channel subcomplexes. Here, we report cryo-EM structures of yeast V-ATPase assembled in vitro from lipid nanodisc reconstituted V and mutant V . Our analysis identified holoenzymes in three active rotary states, indicating that binding of V to V provides sufficient free energy to overcome V autoinhibition. Moreover, the structures suggest that the unequal spacing of V 's proton-carrying glutamic acid residues serves to alleviate the symmetry mismatch between V and V motors, a notion that is supported by mutagenesis experiments. We also uncover a structure of free V bound to Oxr1, a conserved but poorly characterized factor involved in the oxidative stress response. Biochemical experiments show that Oxr1 inhibits V -ATPase and causes disassembly of the holoenzyme, suggesting that Oxr1 plays a direct role in V-ATPase regulation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31541.map.gz emd_31541.map.gz | 185.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31541-v30.xml emd-31541-v30.xml emd-31541.xml emd-31541.xml | 21.7 KB 21.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31541.png emd_31541.png | 175.6 KB | ||
| Filedesc metadata |  emd-31541.cif.gz emd-31541.cif.gz | 7.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31541 http://ftp.pdbj.org/pub/emdb/structures/EMD-31541 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31541 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31541 | HTTPS FTP |
-Related structure data
| Related structure data |  7fdeMC  7fdaC  7fdbC  7fdcC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31541.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31541.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Oxr1 bound V1 subcomplex of Yeast Vacuolar ATPase
+Supramolecule #1: Oxr1 bound V1 subcomplex of Yeast Vacuolar ATPase
+Supramolecule #2: Yeast Vacuolar ATPase subunit C
+Supramolecule #3: Yeast V-type proton ATPase subunits
+Macromolecule #1: V-type proton ATPase subunit C
+Macromolecule #2: V-type proton ATPase subunit E
+Macromolecule #3: V-type proton ATPase subunit G
+Macromolecule #4: Yeast Vacuolar ATPase A subunit
+Macromolecule #5: V-type proton ATPase subunit B
+Macromolecule #6: V-type proton ATPase subunit D
+Macromolecule #7: V-type proton ATPase subunit F
+Macromolecule #8: Oxidation resistance protein 1
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: UltrAuFoil / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: HOMEMADE PLUNGER |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 20447 / Average exposure time: 10.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Algorithm: BACK PROJECTION / Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 20447 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7fde: |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















