+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31087 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | LptB2FGC in complex with LPS from Klebsiella pneumoniae | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationlipopolysaccharide transport / ATP-binding cassette (ABC) transporter complex / transmembrane transport / ATP hydrolysis activity / ATP binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Klebsiella pneumoniae subsp. pneumoniae (bacteria) Klebsiella pneumoniae subsp. pneumoniae (bacteria) | |||||||||
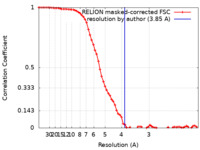
| Method | single particle reconstruction / cryo EM / Resolution: 3.85 Å | |||||||||
 Authors Authors | Luo QS / Shi HG | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Biochem Biophys Res Commun / Year: 2021 Journal: Biochem Biophys Res Commun / Year: 2021Title: Cryo-EM structures of LptBFG and LptBFGC from Klebsiella pneumoniae in complex with lipopolysaccharide. Authors: Qingshan Luo / Huigang Shi / Xueqing Xu /  Abstract: Lipopolysaccharide (LPS) is an essential component of the outer membrane (OM) in most Gram-negative bacteria. LPS transport from the inner membrane (IM) to the OM is achieved by seven ...Lipopolysaccharide (LPS) is an essential component of the outer membrane (OM) in most Gram-negative bacteria. LPS transport from the inner membrane (IM) to the OM is achieved by seven lipopolysaccharide transport proteins (LptA-G). LptBFG, an type VI ATP-binding cassette (ABC) transporter, forms a stable complex with LptC, extracts LPS from the IM and powers LPS transport to the OM. Here we report the cryo-EM structures of LptBFG and LptBFGC from Klebsiella pneumoniae in complex with LPS. The KpLptBFG-LPS structure provides detailed interactions between LPS and the transporter, while the KpLptBFGC-LPS structure may represent an intermediate state that the transmembrane helix of LptC has not been fully inserted into the transmembrane domains of LptBFG. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31087.map.gz emd_31087.map.gz | 25.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31087-v30.xml emd-31087-v30.xml emd-31087.xml emd-31087.xml | 11.9 KB 11.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_31087_fsc.xml emd_31087_fsc.xml | 6.9 KB | Display |  FSC data file FSC data file |
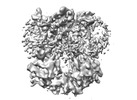
| Images |  emd_31087.png emd_31087.png | 84.2 KB | ||
| Masks |  emd_31087_msk_1.map emd_31087_msk_1.map | 27 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31087 http://ftp.pdbj.org/pub/emdb/structures/EMD-31087 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31087 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31087 | HTTPS FTP |
-Validation report
| Summary document |  emd_31087_validation.pdf.gz emd_31087_validation.pdf.gz | 417.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31087_full_validation.pdf.gz emd_31087_full_validation.pdf.gz | 417.4 KB | Display | |
| Data in XML |  emd_31087_validation.xml.gz emd_31087_validation.xml.gz | 9.2 KB | Display | |
| Data in CIF |  emd_31087_validation.cif.gz emd_31087_validation.cif.gz | 11.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31087 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31087 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31087 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31087 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31087.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31087.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_31087_msk_1.map emd_31087_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : LptB2FGC with LPS from Klebsiella pneumoniae
| Entire | Name: LptB2FGC with LPS from Klebsiella pneumoniae |
|---|---|
| Components |
|
-Supramolecule #1: LptB2FGC with LPS from Klebsiella pneumoniae
| Supramolecule | Name: LptB2FGC with LPS from Klebsiella pneumoniae / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Klebsiella pneumoniae subsp. pneumoniae (bacteria) Klebsiella pneumoniae subsp. pneumoniae (bacteria) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Lipopolysaccharide export system ATP-binding protein LptB
| Macromolecule | Name: Lipopolysaccharide export system ATP-binding protein LptB type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MATLTAKNLA KAYKGRRVVE DVSLTVNSGE IVGLLGPNGA GKTTTFYMVV GIVPRDAGNI IIDDEDISL LPLHARARRG IGYLPQEASI FRRLSVYDNL MAVLQIRDDL TSEQREDRAK E LMEEFHIE HLRDSLGQAL SGGERRRVEI ARALAANPKF ILLDEPFAGV ...String: MATLTAKNLA KAYKGRRVVE DVSLTVNSGE IVGLLGPNGA GKTTTFYMVV GIVPRDAGNI IIDDEDISL LPLHARARRG IGYLPQEASI FRRLSVYDNL MAVLQIRDDL TSEQREDRAK E LMEEFHIE HLRDSLGQAL SGGERRRVEI ARALAANPKF ILLDEPFAGV DPISVIDIKR II EHLRDSG LGVLITDHNV RETLAVCERA YIVSQGHLIA HGTPQQILED EQVKRVYLGE DFR L |
-Macromolecule #2: Lipopolysaccharide export system permease protein LptF
| Macromolecule | Name: Lipopolysaccharide export system permease protein LptF type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MIIIRYLVRE TLKSQLAILF ILLLIFFCQK LVRILGAAVD GDIPTNLVLS LLGLGIPEMA QLILPLSLF LGLLMTLGKL YTESEITVMH ACGLSKAVLI KAAMILALFT GAVAAVNVMW A GPWSSRHQ DEVLAEAKAN PGMAALAQGQ FQQASDGNAV MFIESVNGNR ...String: MIIIRYLVRE TLKSQLAILF ILLLIFFCQK LVRILGAAVD GDIPTNLVLS LLGLGIPEMA QLILPLSLF LGLLMTLGKL YTESEITVMH ACGLSKAVLI KAAMILALFT GAVAAVNVMW A GPWSSRHQ DEVLAEAKAN PGMAALAQGQ FQQASDGNAV MFIESVNGNR FHDVFLAQLR PK GNARPSV VVADSGELSQ QKDGSQVVTL NKGTRFEGTA MLRDFRITDF NNYQAIIGHQ AVS ADPDDT EQMDMRTLWK THTDRARAEL HWRFTLVATV FIMALMVVPL SVVNPRQGRV LSML PAMLL YLVFFLLQTS IKSNGGKGKM DPAIWMWAIN LLYFALAVLL NLWDTVPMRR FRARF NKGA A |
-Macromolecule #3: LPS export ABC transporter permease LptG
| Macromolecule | Name: LPS export ABC transporter permease LptG / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MQAFGVLDRY IGKTIFNTIM MTLFMLVSLS GIIKFVDQLK KSGQGSYDAL GAGLYTILSV PKDIQIFFP MAALLGALLG LGMLAQRSEL VVMQASGFTR LQVALAVMKT AIPLVLLTMA I GEWVAPQG EQMARNYRAQ QMYGGSLLST QQGLWAKDGH NFVYIERVKG ...String: MQAFGVLDRY IGKTIFNTIM MTLFMLVSLS GIIKFVDQLK KSGQGSYDAL GAGLYTILSV PKDIQIFFP MAALLGALLG LGMLAQRSEL VVMQASGFTR LQVALAVMKT AIPLVLLTMA I GEWVAPQG EQMARNYRAQ QMYGGSLLST QQGLWAKDGH NFVYIERVKG NDELGGVSIY AF NPERRLQ SVRYAASAKF DSENKVWRLS QVDESDLTDP KQVTGSQMVS GTWKTNLTPD KLG VVALDP DALSISGLHN YVKYLKSSGQ DPGRYQLNMW SKIFQPLSVA VMMLMALSFI FGPL RSVPM GVRVVTGISF GFIFYVLDQI FGPLTLVYGI PPIIGALLPS ASFFLISLWL MMRKA |
-Macromolecule #4: Lipopolysaccharide export system protein LptC
| Macromolecule | Name: Lipopolysaccharide export system protein LptC / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MSKTRRWVII LLSLLALILI GLNLANTDDT AQPEVNPNDP TYKSEHTDTV VYSPEGALSY RLIAEHVEY FSDQEVSWFT KPVMTTFDTN KVPTWSVRAD KAKLTNDRML YLYGHVEVNA L APDSQLRK ITTDNAQINL VTQDVTSDDM VTLYGTTFNS SGLKMRGNLR ...String: MSKTRRWVII LLSLLALILI GLNLANTDDT AQPEVNPNDP TYKSEHTDTV VYSPEGALSY RLIAEHVEY FSDQEVSWFT KPVMTTFDTN KVPTWSVRAD KAKLTNDRML YLYGHVEVNA L APDSQLRK ITTDNAQINL VTQDVTSDDM VTLYGTTFNS SGLKMRGNLR SKNAELIEKV RT SYEIQNK QTQP |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: NITROGEN / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K2 BASE (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.4000000000000001 µm |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)