+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30609 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human ABCB6 transporter | ||||||||||||
 Map data Map data | map for the full-length ABCB6 | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Transporter / Dimer / Porphyrins / Heme / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationDefective ABCB6 causes MCOPCB7 / cellular detoxification of cadmium ion / Mitochondrial ABC transporters / tetrapyrrole metabolic process / ABC-type heme transporter / porphyrin-containing compound metabolic process / tetrapyrrole binding / heme metabolic process / heme transport / porphyrin-containing compound biosynthetic process ...Defective ABCB6 causes MCOPCB7 / cellular detoxification of cadmium ion / Mitochondrial ABC transporters / tetrapyrrole metabolic process / ABC-type heme transporter / porphyrin-containing compound metabolic process / tetrapyrrole binding / heme metabolic process / heme transport / porphyrin-containing compound biosynthetic process / melanosome assembly / ABC-type heme transporter activity / heme transmembrane transport / melanosome membrane / multivesicular body membrane / mitochondrial envelope / endolysosome membrane / vacuolar membrane / skin development / efflux transmembrane transporter activity / intracellular copper ion homeostasis / ABC-type transporter activity / ATP-binding cassette (ABC) transporter complex / brain development / transmembrane transport / early endosome membrane / intracellular iron ion homeostasis / mitochondrial outer membrane / endosome / Golgi membrane / lysosomal membrane / heme binding / endoplasmic reticulum membrane / endoplasmic reticulum / Golgi apparatus / ATP hydrolysis activity / mitochondrion / extracellular exosome / nucleoplasm / ATP binding / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / Resolution: 5.2 Å | ||||||||||||
 Authors Authors | Wang C / Cao C | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Protein Sci / Year: 2020 Journal: Protein Sci / Year: 2020Title: Cryo-electron microscopy structure of human ABCB6 transporter. Authors: Chunyu Wang / Can Cao / Nan Wang / Xiangxi Wang / Xianping Wang / Xuejun C Zhang /  Abstract: Human ATP-binding cassette transporter 6 of subfamily B (ABCB6) is an ABC transporter involved in the translocation toxic metals and anti-cancer drugs. Using cryo-electron microscopy, we determined ...Human ATP-binding cassette transporter 6 of subfamily B (ABCB6) is an ABC transporter involved in the translocation toxic metals and anti-cancer drugs. Using cryo-electron microscopy, we determined the molecular structure of full-length ABCB6 in an apo state. The structure of ABCB6 unravels the architecture of a full-length ABCB transporter that harbors two N-terminal transmembrane domains which is indispensable for its ATPase activity in our in vitro assay. A slit-like substrate binding pocket of ABCB6 may accommodate the planar shape of porphyrins, and the existence of a secondary cavity near the mitochondrial intermembrane space side would further facilitate substrate release. Furthermore, the ATPase activity of ABCB6 stimulated with a variety of porphyrin substrates showed different profiles in the presence of glutathione (GSH), suggesting the action of a distinct substrate translocation mechanism depending on the use of GSH as a cofactor. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30609.map.gz emd_30609.map.gz | 62.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30609-v30.xml emd-30609-v30.xml emd-30609.xml emd-30609.xml | 11 KB 11 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30609_fsc.xml emd_30609_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_30609.png emd_30609.png | 11.6 KB | ||
| Filedesc metadata |  emd-30609.cif.gz emd-30609.cif.gz | 5.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30609 http://ftp.pdbj.org/pub/emdb/structures/EMD-30609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30609 | HTTPS FTP |
-Related structure data
| Related structure data |  7d7nMC  7d7rC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30609.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30609.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map for the full-length ABCB6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
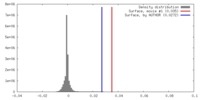
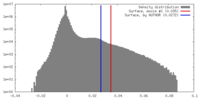
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human ABCB6 protein homodimer.
| Entire | Name: Human ABCB6 protein homodimer. |
|---|---|
| Components |
|
-Supramolecule #1: Human ABCB6 protein homodimer.
| Supramolecule | Name: Human ABCB6 protein homodimer. / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: ATP-binding cassette sub-family B member 6, mitochondrial
| Macromolecule | Name: ATP-binding cassette sub-family B member 6, mitochondrial type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 93.974172 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MVTVGNYCEA EGPVGPAWMQ DGLSPCFFFT LVPSTRMALG TLALVLALPC RRRERPAGAD SLSWGAGPRI SPYVLQLLLA TLQAALPLA GLAGRVGTAR GAPLPSYLLL ASVLESLAGA CGLWLLVVER SQARQRLAMG IWIKFRHSPG LLLLWTVAFA A ENLALVSW ...String: MVTVGNYCEA EGPVGPAWMQ DGLSPCFFFT LVPSTRMALG TLALVLALPC RRRERPAGAD SLSWGAGPRI SPYVLQLLLA TLQAALPLA GLAGRVGTAR GAPLPSYLLL ASVLESLAGA CGLWLLVVER SQARQRLAMG IWIKFRHSPG LLLLWTVAFA A ENLALVSW NSPQWWWARA DLGQQVQFSL WVLRYVVSGG LFVLGLWAPG LRPQSYTLQV HEEDQDVERS QVRSAAQQST WR DFGRKLR LLSGYLWPRG SPALQLVVLI CLGLMGLERA LNVLVPIFYR NIVNLLTEKA PWNSLAWTVT SYVFLKFLQG GGT GSTGFV SNLRTFLWIR VQQFTSRRVE LLIFSHLHEL SLRWHLGRRT GEVLRIADRG TSSVTGLLSY LVFNVIPTLA DIII GIIYF SMFFNAWFGL IVFLCMSLYL TLTIVVTEWR TKFRRAMNTQ ENATRARAVD SLLNFETVKY YNAESYEVER YREAI IKYQ GLEWKSSASL VLLNQTQNLV IGLGLLAGSL LCAYFVTEQK LQVGDYVLFG TYIIQLYMPL NWFGTYYRMI QTNFID MEN MFDLLKEETE VKDLPGAGPL RFQKGRIEFE NVHFSYADGR ETLQDVSFTV MPGQTLALVG PSGAGKSTIL RLLFRFY DI SSGCIRIDGQ DISQVTQASL RSHIGVVPQD TVLFNDTIAD NIRYGRVTAG NDEVEAAAQA AGIHDAIMAF PEGYRTQV G ERGLKLSGGE KQRVAIARTI LKAPGIILLD EATSALDTSN ERAIQASLAK VCANRTTIVV AHRLSTVVNA DQILVIKDG CIVERGRHEA LLSRGGVYAD MWQLQQGQEE TSEDTKPQTM ER UniProtKB: ATP-binding cassette sub-family B member 6 |
-Experimental details
-Structure determination
 Processing Processing | single particle reconstruction |
|---|---|
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)