[English] 日本語
 Yorodumi
Yorodumi- EMDB-3045: Density map of the scanning state of the mammalian SRP-ribosome c... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3045 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Density map of the scanning state of the mammalian SRP-ribosome complex | |||||||||
 Map data Map data | This is the final masked and sharpened reconstruction | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ribosomes / SRP / mammal / translocation | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Voorhees RM / Hegde RS | |||||||||
 Citation Citation |  Journal: Elife / Year: 2015 Journal: Elife / Year: 2015Title: Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. Authors: Rebecca M Voorhees / Ramanujan S Hegde /  Abstract: The universally conserved signal recognition particle (SRP) is essential for the biogenesis of most integral membrane proteins. SRP scans the nascent chains of translating ribosomes, preferentially ...The universally conserved signal recognition particle (SRP) is essential for the biogenesis of most integral membrane proteins. SRP scans the nascent chains of translating ribosomes, preferentially engaging those with hydrophobic targeting signals, and delivers these ribosome-nascent chain complexes to the membrane. Here, we present structures of native mammalian SRP-ribosome complexes in the scanning and engaged states. These structures reveal the near-identical SRP architecture of these two states, show many of the SRP-ribosome interactions at atomic resolution, and suggest how the polypeptide-binding M domain selectively engages hydrophobic signals. The scanning M domain, pre-positioned at the ribosomal exit tunnel, is auto-inhibited by a C-terminal amphipathic helix occluding its hydrophobic binding groove. Upon engagement, the hydrophobic targeting signal displaces this amphipathic helix, which then acts as a protective lid over the signal. Biochemical experiments suggest how scanning and engagement are coordinated with translation elongation to minimize exposure of hydrophobic signals during membrane targeting. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3045.map.gz emd_3045.map.gz | 31.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3045-v30.xml emd-3045-v30.xml emd-3045.xml emd-3045.xml | 8.4 KB 8.4 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-3045.png EMD-3045.png | 354.4 KB | ||
| Others |  emd_3045_half_map_1.map.gz emd_3045_half_map_1.map.gz emd_3045_half_map_2.map.gz emd_3045_half_map_2.map.gz | 224.9 MB 224.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3045 http://ftp.pdbj.org/pub/emdb/structures/EMD-3045 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3045 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3045 | HTTPS FTP |
-Related structure data
| Related structure data |  3janMC  3037C  3046C  3jajC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3045.map.gz / Format: CCP4 / Size: 276 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3045.map.gz / Format: CCP4 / Size: 276 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is the final masked and sharpened reconstruction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.34 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
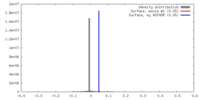
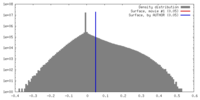
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Supplemental map: emd 3045 half map 1.map
| File | emd_3045_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
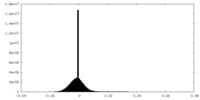
| Density Histograms |
-Supplemental map: emd 3045 half map 2.map
| File | emd_3045_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
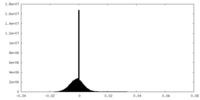
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of the mammalian ribosome and SRP in its scanning mode
| Entire | Name: Complex of the mammalian ribosome and SRP in its scanning mode |
|---|---|
| Components |
|
-Supramolecule #1000: Complex of the mammalian ribosome and SRP in its scanning mode
| Supramolecule | Name: Complex of the mammalian ribosome and SRP in its scanning mode type: sample / ID: 1000 / Oligomeric state: Heterodimer of mammalian ribosome and SRP / Number unique components: 2 |
|---|
-Supramolecule #1: Mammalian ribosome
| Supramolecule | Name: Mammalian ribosome / type: complex / ID: 1 / Name.synonym: 80S ribosome / Recombinant expression: No / Ribosome-details: ribosome-eukaryote: ALL |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Signal recognition particle
| Macromolecule | Name: Signal recognition particle / type: rna / ID: 1 / Name.synonym: SRP / Details: ribonucleoprotein complex / Classification: OTHER / Structure: OTHER / Synthetic?: No |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: 50 mM HEPES pH 7.5, 200 mM 469 KAc, 15 mM MgAc2, and 1 mM DTT |
|---|---|
| Grid | Details: glow-discharged holey carbon grids (Quantifoil R2/2), coated with a ~70 angstrom thick layer of amorphous carbon |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV Method: sample was applied to the grid, followed by a 30 s incubation at 4C, 3 s of blotting |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Date | Nov 14, 2014 |
| Image recording | Category: CCD / Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 27 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | The particles were selected using the automated particle picker in Relion |
|---|---|
| CTF correction | Details: Each particle |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.9 Å / Resolution method: OTHER / Software - Name: Relion Details: Final maps were calculated from two averaged data sets Number images used: 27627 |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)