[English] 日本語
 Yorodumi
Yorodumi- EMDB-30100: 3.4A apoferritin from JEM-2100F + K2 Summit at 40K, RELION 3.0 pr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30100 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 3.4A apoferritin from JEM-2100F + K2 Summit at 40K, RELION 3.0 processing, manual acquisition. | |||||||||
 Map data Map data | 3.4A apoferritin, JEM-2100F K2 Summit, 40K, RELION 3, manual acqusition, postprocessed map. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationautolysosome / ferric iron binding / iron ion transport / cytoplasmic vesicle / intracellular iron ion homeostasis Similarity search - Function | |||||||||
| Biological species |  | |||||||||
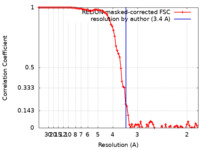
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Burton-Smith RN / Kayama Y / Song C / Kato T / Murata K | |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2021 Journal: Sci Rep / Year: 2021Title: Below 3 Å structure of apoferritin using a multipurpose TEM with a side entry cryoholder. Authors: Yoko Kayama / Raymond N Burton-Smith / Chihong Song / Naoya Terahara / Takayuki Kato / Kazuyoshi Murata /  Abstract: Recently, the structural analysis of protein complexes by cryo-electron microscopy (cryo-EM) single particle analysis (SPA) has had great impact as a biophysical method. Many results of cryo-EM SPA ...Recently, the structural analysis of protein complexes by cryo-electron microscopy (cryo-EM) single particle analysis (SPA) has had great impact as a biophysical method. Many results of cryo-EM SPA are based on data acquired on state-of-the-art cryo-electron microscopes customized for SPA. These are currently only available in limited locations around the world, where securing machine time is highly competitive. One potential solution for this time-competitive situation is to reuse existing multi-purpose equipment, although this comes with performance limitations. Here, a multi-purpose TEM with a side entry cryo-holder was used to evaluate the potential of high-resolution SPA, resulting in a 3 Å resolution map of apoferritin with local resolution extending to 2.6 Å. This map clearly showed two positions of an aromatic side chain. Further, examination of optimal imaging conditions depending on two different multi-purpose electron microscope and camera combinations was carried out, demonstrating that higher magnifications are not always necessary or desirable. Since automation is effectively a requirement for large-scale data collection, and augmenting the multi-purpose equipment is possible, we expanded testing by acquiring data with SerialEM using a β-galactosidase test sample. This study demonstrates the possibilities of more widely available and established electron microscopes, and their applications for cryo-EM SPA. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30100.map.gz emd_30100.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30100-v30.xml emd-30100-v30.xml emd-30100.xml emd-30100.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30100_fsc.xml emd_30100_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_30100.png emd_30100.png | 103.2 KB | ||
| Masks |  emd_30100_msk_1.map emd_30100_msk_1.map | 64 MB |  Mask map Mask map | |
| Others |  emd_30100_half_map_1.map.gz emd_30100_half_map_1.map.gz emd_30100_half_map_2.map.gz emd_30100_half_map_2.map.gz | 46 MB 45.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30100 http://ftp.pdbj.org/pub/emdb/structures/EMD-30100 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30100 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30100 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30100.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30100.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3.4A apoferritin, JEM-2100F K2 Summit, 40K, RELION 3, manual acqusition, postprocessed map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
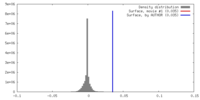
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_30100_msk_1.map emd_30100_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
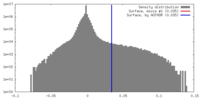
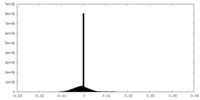
| Density Histograms |
-Half map: 3.4A apoferritin, JEM-2100F K2 Summit, 40K, RELION 3,...
| File | emd_30100_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3.4A apoferritin, JEM-2100F K2 Summit, 40K, RELION 3, manual acqusition, half map 1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
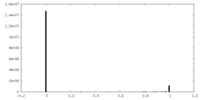
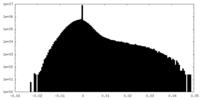
| Density Histograms |
-Half map: 3.4A apoferritin, JEM-2100F K2 Summit, 40K, RELION 3,...
| File | emd_30100_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3.4A apoferritin, JEM-2100F K2 Summit, 40K, RELION 3, manual acqusition, half map 2. | ||||||||||||
| Projections & Slices |
| ||||||||||||
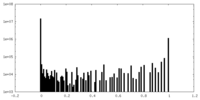
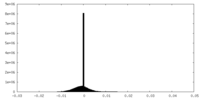
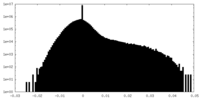
| Density Histograms |
- Sample components
Sample components
-Entire : Apoferritin, 3.4A, JEM-2100F + K2 Summit, 40K, RELION 3.0
| Entire | Name: Apoferritin, 3.4A, JEM-2100F + K2 Summit, 40K, RELION 3.0 |
|---|---|
| Components |
|
-Supramolecule #1: Apoferritin, 3.4A, JEM-2100F + K2 Summit, 40K, RELION 3.0
| Supramolecule | Name: Apoferritin, 3.4A, JEM-2100F + K2 Summit, 40K, RELION 3.0 type: complex / ID: 1 / Parent: 0 / Details: Octahedral symmetry. |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 500 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: MOLYBDENUM / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2100F |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 5.0 sec. / Average electron dose: 46.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal magnification: 40000 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)