[English] 日本語
 Yorodumi
Yorodumi- EMDB-29487: Pseudomonas phage E217 neck (portal, head-to-tail connector, coll... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pseudomonas phage E217 neck (portal, head-to-tail connector, collar and gateway proteins) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | pseudomonas / phage / E217 / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationInorganic pyrophosphatase domain / : / : / Inorganic Pyrophosphatase / E217 collar protein gp28 / E217 gateway protein gp29 / Protein of unknown function DUF4054 / Protein of unknown function (DUF4054) / Protein of unknown function DUF1073 / Phage portal protein Similarity search - Domain/homology | |||||||||
| Biological species |  Pseudomonas phage vB_PaeM_E217 (virus) Pseudomonas phage vB_PaeM_E217 (virus) | |||||||||
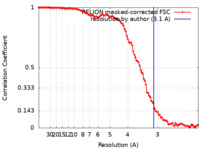
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Li F / Cingolani G / Hou C | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: High-resolution cryo-EM structure of the Pseudomonas bacteriophage E217. Authors: Fenglin Li / Chun-Feng David Hou / Ravi K Lokareddy / Ruoyu Yang / Francesca Forti / Federica Briani / Gino Cingolani /   Abstract: E217 is a Pseudomonas phage used in an experimental cocktail to eradicate cystic fibrosis-associated Pseudomonas aeruginosa. Here, we describe the structure of the whole E217 virion before and after ...E217 is a Pseudomonas phage used in an experimental cocktail to eradicate cystic fibrosis-associated Pseudomonas aeruginosa. Here, we describe the structure of the whole E217 virion before and after DNA ejection at 3.1 Å and 4.5 Å resolution, respectively, determined using cryogenic electron microscopy (cryo-EM). We identify and build de novo structures for 19 unique E217 gene products, resolve the tail genome-ejection machine in both extended and contracted states, and decipher the complete architecture of the baseplate formed by 66 polypeptide chains. We also determine that E217 recognizes the host O-antigen as a receptor, and we resolve the N-terminal portion of the O-antigen-binding tail fiber. We propose that E217 design principles presented in this paper are conserved across PB1-like Myoviridae phages of the Pbunavirus genus that encode a ~1.4 MDa baseplate, dramatically smaller than the coliphage T4. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29487.map.gz emd_29487.map.gz | 407.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29487-v30.xml emd-29487-v30.xml emd-29487.xml emd-29487.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29487_fsc.xml emd_29487_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_29487.png emd_29487.png | 110 KB | ||
| Filedesc metadata |  emd-29487.cif.gz emd-29487.cif.gz | 6.1 KB | ||
| Others |  emd_29487_half_map_1.map.gz emd_29487_half_map_1.map.gz emd_29487_half_map_2.map.gz emd_29487_half_map_2.map.gz | 410.3 MB 411.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29487 http://ftp.pdbj.org/pub/emdb/structures/EMD-29487 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29487 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29487 | HTTPS FTP |
-Related structure data
| Related structure data |  8fvhMC  8envC  8eonC  8frsC  8fuvC  8fvgC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_29487.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29487.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.12 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29487_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_29487_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Pseudomonas phage vB_PaeM_E217
| Entire | Name:  Pseudomonas phage vB_PaeM_E217 (virus) Pseudomonas phage vB_PaeM_E217 (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Pseudomonas phage vB_PaeM_E217
| Supramolecule | Name: Pseudomonas phage vB_PaeM_E217 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 2034346 / Sci species name: Pseudomonas phage vB_PaeM_E217 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: Yes / Virus empty: Yes |
|---|
-Macromolecule #1: E217 collar protein gp28
| Macromolecule | Name: E217 collar protein gp28 / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage vB_PaeM_E217 (virus) Pseudomonas phage vB_PaeM_E217 (virus) |
| Molecular weight | Theoretical: 14.234122 KDa |
| Sequence | String: IPGANLLRMA FGVIGTQIVR YRKFEQRVKN DQAQYVSMFG EPFDLAASVQ RVRRDQYAQF NLEFQRNYVM IFANFDMVDL DRNMAGDQF LWTGRVFQLE SQGSWFYQDG WGVCLAVDIG AAKA UniProtKB: Virion protein |
-Macromolecule #2: E217 gateway protein gp29
| Macromolecule | Name: E217 gateway protein gp29 / type: protein_or_peptide / ID: 2 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage vB_PaeM_E217 (virus) Pseudomonas phage vB_PaeM_E217 (virus) |
| Molecular weight | Theoretical: 21.057492 KDa |
| Sequence | String: MFDGELIAKL VVELNAAMTS AQEALQFPDF EVVQKAQPTQ QGTSTRPTIF FQKLFDIPRG WPATDWHLDN TARKYVEITR QHVETTFQI SSLHWQNPEI THVVTASDIA NYVRAYFQAR STIERVKELD FLILRVSQIS NEAFENDNHQ FEFHPSFDMV V TYNQYIRL YENAAYSADG VLIG UniProtKB: Phage protein |
-Macromolecule #3: E217 head-to-tail connector protein gp27
| Macromolecule | Name: E217 head-to-tail connector protein gp27 / type: protein_or_peptide / ID: 3 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage vB_PaeM_E217 (virus) Pseudomonas phage vB_PaeM_E217 (virus) |
| Molecular weight | Theoretical: 16.885285 KDa |
| Sequence | String: MVIFDEHKFR TLFPEFADPA AYPDVRLQMY FDIACEFISD RDSPYRILNG KALEACLYLL TAHLLSLSTM QVQGAAGGGV TAGGTQGGF ITSATVGEVS VAKLAPPAKN GWQWWLSGTP YGQELWALLS VKAVGGFYIG GLPERRGFRK VGGTFW UniProtKB: Uncharacterized protein |
-Macromolecule #4: E217 portal protein gp19
| Macromolecule | Name: E217 portal protein gp19 / type: protein_or_peptide / ID: 4 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage vB_PaeM_E217 (virus) Pseudomonas phage vB_PaeM_E217 (virus) |
| Molecular weight | Theoretical: 48.635984 KDa |
| Sequence | String: PTMLQDWYNS QGFIGYQACA IISQHWLVDK ACSMSGEDAA RNGWELKSDG RKLSDEQSAL IARRDMEFRV KDNLVELNRF KNVFGVRIA LFVVESDDPD YYEKPFNPDG VTPGSYKGIS QIDPYWAMPQ LTAGSTADPS SEHFYEPDFW IISGKKYHRS H LVVVRGPQ ...String: PTMLQDWYNS QGFIGYQACA IISQHWLVDK ACSMSGEDAA RNGWELKSDG RKLSDEQSAL IARRDMEFRV KDNLVELNRF KNVFGVRIA LFVVESDDPD YYEKPFNPDG VTPGSYKGIS QIDPYWAMPQ LTAGSTADPS SEHFYEPDFW IISGKKYHRS H LVVVRGPQ PPDILKPTYI FGGIPLTQRI YERVYAAERT ANEAPLLAMS KRTSTIHVDV EKAIANEEAF NARLAFWIAN RD NHGVKVL GIDEGMEQFD TNLADFDSII MNQYQLVAAI AKTPATKLLG TSPKGFNATG EHETISYHEE LESIQEHIFD PLL ERHYLL LAKSEEIDVQ LEIVWNPVDS TSSQQQAELN NKKAATDEIY INSGVVSPDE VRERLRDDPR SGYNRLTDDQ AETE PGMSP ENLAEFEKAG AQSAKAKGEA ERAEAQAG UniProtKB: Uncharacterized protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 81000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)