[English] 日本語
 Yorodumi
Yorodumi- EMDB-25586: CryoEM structure of somatostatin receptor 2 in complex with Octre... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25586 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
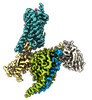
| Title | CryoEM structure of somatostatin receptor 2 in complex with Octreotide and Gi3. | |||||||||
 Map data Map data | CryoEM structure of SSTR2/Gi3 complex bound to SST14 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / Receptor / Complex / MEMBRANE PROTEIN / MEMBRANE PROTEIN-SIGNALING PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsomatostatin signaling pathway / response to acrylamide / adenylate cyclase-inhibiting opioid receptor signaling pathway / dynorphin receptor activity / regulation of saliva secretion / MECP2 regulates transcription of neuronal ligands / negative regulation of luteinizing hormone secretion / sensory perception of temperature stimulus / somatostatin receptor activity / hormone-mediated apoptotic signaling pathway ...somatostatin signaling pathway / response to acrylamide / adenylate cyclase-inhibiting opioid receptor signaling pathway / dynorphin receptor activity / regulation of saliva secretion / MECP2 regulates transcription of neuronal ligands / negative regulation of luteinizing hormone secretion / sensory perception of temperature stimulus / somatostatin receptor activity / hormone-mediated apoptotic signaling pathway / positive regulation of eating behavior / G protein-coupled opioid receptor activity / G protein-coupled opioid receptor signaling pathway / positive regulation of dopamine secretion / negative regulation of adenylate cyclase activity / sensory perception / response to acidic pH / maternal behavior / positive regulation of potassium ion transmembrane transport / receptor serine/threonine kinase binding / positive regulation of p38MAPK cascade / neuropeptide binding / response to steroid hormone / hyperosmotic response / GTP metabolic process / eating behavior / cellular response to glucocorticoid stimulus / positive regulation of macroautophagy / conditioned place preference / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / response to amino acid / neuropeptide signaling pathway / neuronal dense core vesicle / estrous cycle / regulation of postsynaptic membrane neurotransmitter receptor levels / digestion / MECP2 regulates neuronal receptors and channels / behavioral response to cocaine / Adenylate cyclase inhibitory pathway / axon terminus / response to nutrient / sensory perception of pain / T-tubule / regulation of cell migration / Peptide ligand-binding receptors / sarcoplasmic reticulum / PDZ domain binding / response to nicotine / locomotory behavior / cellular response to estradiol stimulus / cellular response to glucose stimulus / hormone activity / G protein-coupled receptor binding / response to insulin / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / GABA-ergic synapse / response to estrogen / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / synaptic vesicle membrane / GDP binding / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / cell-cell signaling / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Robertson MJ / Skinotis G | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Plasticity in ligand recognition at somatostatin receptors. Authors: Michael J Robertson / Justin G Meyerowitz / Ouliana Panova / Kenneth Borrelli / Georgios Skiniotis /  Abstract: Somatostatin is a signaling peptide that plays a pivotal role in physiologic processes relating to metabolism and growth through its actions at somatostatin receptors (SSTRs). Members of the SSTR ...Somatostatin is a signaling peptide that plays a pivotal role in physiologic processes relating to metabolism and growth through its actions at somatostatin receptors (SSTRs). Members of the SSTR subfamily, particularly SSTR2, are key drug targets for neuroendocrine neoplasms, with synthetic peptide agonists currently in clinical use. Here, we show the cryogenic-electron microscopy structures of active-state SSTR2 in complex with heterotrimeric G and either the endogenous ligand SST14 or the FDA-approved drug octreotide. Complemented by biochemical assays and molecular dynamics simulations, these structures reveal key details of ligand recognition and receptor activation at SSTRs. We find that SSTR ligand recognition is highly diverse, as demonstrated by ligand-induced conformational changes in ECL2 and substantial sequence divergence across subtypes in extracellular regions. Despite this complexity, we rationalize several known sources of SSTR subtype selectivity and identify an additional interaction for specific binding. These results provide valuable insights for structure-based drug discovery at SSTRs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25586.map.gz emd_25586.map.gz | 168 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25586-v30.xml emd-25586-v30.xml emd-25586.xml emd-25586.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25586.png emd_25586.png | 130.2 KB | ||
| Filedesc metadata |  emd-25586.cif.gz emd-25586.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25586 http://ftp.pdbj.org/pub/emdb/structures/EMD-25586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25586 | HTTPS FTP |
-Related structure data
| Related structure data |  7t10MC  7t11C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10931 (Title: Cryogenic electron microscopy of somatostatin receptor 2/SST14/Gi3 complex EMPIAR-10931 (Title: Cryogenic electron microscopy of somatostatin receptor 2/SST14/Gi3 complexData size: 4.5 TB Data #1: Raw movies of SSTR2/SST14/Gi3 complex collected on a K3 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25586.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25586.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM structure of SSTR2/Gi3 complex bound to SST14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8521 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Somatostatin receptor 2 in complex with Somatostatin-14 and Gi3.
| Entire | Name: Somatostatin receptor 2 in complex with Somatostatin-14 and Gi3. |
|---|---|
| Components |
|
-Supramolecule #1: Somatostatin receptor 2 in complex with Somatostatin-14 and Gi3.
| Supramolecule | Name: Somatostatin receptor 2 in complex with Somatostatin-14 and Gi3. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|
-Macromolecule #1: Somatostatin receptor type 2,Kappa-type opioid receptor
| Macromolecule | Name: Somatostatin receptor type 2,Kappa-type opioid receptor type: protein_or_peptide / ID: 1 Details: Somatostatin receptor type 2 with intracellular loop 3 (ICL3) replaced with the ICL3 from the Kappa-type opioid receptor Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.784211 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DYKDDDDAMG QPGNGSAFLL APNRSHAPDH DVENLYFQGM DMADEPLNGS HTWLSIPFDL NGSVVSTNTS NQTEPYYDLT SNAVLTFIY FVVCIIGLCG NTLVIYVILR YAKMKTITNI YILNLAIADE LFMLGLPFLA MQVALVHWPF GKAICRVVMT V DGINQFTS ...String: DYKDDDDAMG QPGNGSAFLL APNRSHAPDH DVENLYFQGM DMADEPLNGS HTWLSIPFDL NGSVVSTNTS NQTEPYYDLT SNAVLTFIY FVVCIIGLCG NTLVIYVILR YAKMKTITNI YILNLAIADE LFMLGLPFLA MQVALVHWPF GKAICRVVMT V DGINQFTS IFCLTVMSID RYLAVVHPIK SAKWRRPRTA KMITMAVWGV SLLVILPIMI YAGLRSNQWG RSSCTINWPG ES GAWYTGF IIYTFILGFL VPLTIICLCY LFIIIKVKSV RLLSGSREKD RNLRKVTRMV SIVVAVFIFC WLPFYIFNVS SVS MAISPT PALKGMFDFV VVLTYANSCA NPILYAFLSD NFKKSFQNVL CLVKVSGTDD GERSDSKQDK SRLNETTETQ RTLL NGDLQ TSI UniProtKB: Somatostatin receptor type 2, Kappa-type opioid receptor, Somatostatin receptor type 2 |
-Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-3
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-3 type: protein_or_peptide / ID: 2 / Details: dominant negative / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.584156 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AKEVKLLLLG AGESGKNTIV KQMKIIHEDG YSEDECKQYK VVVYSNTIQS IIAIIRAMG RLKIDFGEAA RADDARQLFV LAGSAEEGVM TPELAGVIKR LWRDGGVQAC FSRSREYQLN DSASYYLNDL D RISQSNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AKEVKLLLLG AGESGKNTIV KQMKIIHEDG YSEDECKQYK VVVYSNTIQS IIAIIRAMG RLKIDFGEAA RADDARQLFV LAGSAEEGVM TPELAGVIKR LWRDGGVQAC FSRSREYQLN DSASYYLNDL D RISQSNYI PTQQDVLRTR VKTTGIVETH FTFKDLYFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHASM KLFDSICNNK WFTETSIILF LNKKDLFEEK IKRSPLTICY PEYTGSNTYE EAAAYIQCQF EDLNRRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKECGLY UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-3 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.671102 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: PGSSGSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQD GKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY LSCCRFLDDN Q IVTSSGDT ...String: PGSSGSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQD GKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY LSCCRFLDDN Q IVTSSGDT TCALWDIETG QQTTTFTGHT GDVMSLSLAP DTRLFVSGAC DASAKLWDVR EGMCRQTFTG HESDINAICF FP NGNAFAT GSDDATCRLF DLRADQELMT YSHDNIICGI TSVSFSKSGR LLLAGYDDFN CNVWDALKAD RAGVLAGHDN RVS CLGVTD DGMAVATGSW DSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: Somatostatin-14
| Macromolecule | Name: Somatostatin-14 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.641909 KDa |
| Sequence | String: AGCKNFFWKT FTSC UniProtKB: Somatostatin |
-Macromolecule #6: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 27.784896 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL KAAAHHHHHH HH |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 5 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.04 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: ab initio |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 442863 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















