[English] 日本語
 Yorodumi
Yorodumi- EMDB-25186: Asymmetric reconstruction of membrane-bound HIV Env from virus-li... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25186 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetric reconstruction of membrane-bound HIV Env from virus-like particles | ||||||||||||
 Map data Map data | Asymmetric reconstruction of HIV Env glycoprotein (ADA.CM.v4) on virus-like particle membrane | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | HIV-1 / envelope protein / glycoprotein / Env / membrane bound / VIRAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell ...symbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / membrane Similarity search - Function | ||||||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||||||||
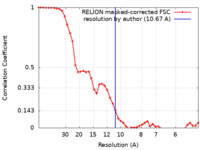
| Method | subtomogram averaging / cryo EM / Resolution: 10.67 Å | ||||||||||||
 Authors Authors | Mangala Prasad V / Lee KK | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Cryo-ET of Env on intact HIV virions reveals structural variation and positioning on the Gag lattice. Authors: Vidya Mangala Prasad / Daniel P Leaman / Klaus N Lovendahl / Jacob T Croft / Mark A Benhaim / Edgar A Hodge / Michael B Zwick / Kelly K Lee /  Abstract: HIV-1 Env mediates viral entry into host cells and is the sole target for neutralizing antibodies. However, Env structure and organization in its native virion context has eluded detailed ...HIV-1 Env mediates viral entry into host cells and is the sole target for neutralizing antibodies. However, Env structure and organization in its native virion context has eluded detailed characterization. Here, we used cryo-electron tomography to analyze Env in mature and immature HIV-1 particles. Immature particles showed distinct Env positioning relative to the underlying Gag lattice, providing insights into long-standing questions about Env incorporation. A 9.1-Å sub-tomogram-averaged reconstruction of virion-bound Env in conjunction with structural mass spectrometry revealed unexpected features, including a variable central core of the gp41 subunit, heterogeneous glycosylation between protomers, and a flexible stalk that allows Env tilting and variable exposure of neutralizing epitopes. Together, our results provide an integrative understanding of HIV assembly and structural variation in Env antigen presentation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25186.map.gz emd_25186.map.gz | 535.8 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25186-v30.xml emd-25186-v30.xml emd-25186.xml emd-25186.xml | 14.5 KB 14.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25186_fsc.xml emd_25186_fsc.xml | 4.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_25186.png emd_25186.png | 70.1 KB | ||
| Filedesc metadata |  emd-25186.cif.gz emd-25186.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25186 http://ftp.pdbj.org/pub/emdb/structures/EMD-25186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25186 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25186.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25186.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric reconstruction of HIV Env glycoprotein (ADA.CM.v4) on virus-like particle membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.58 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human immunodeficiency virus 1
| Entire | Name:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
|---|---|
| Components |
|
-Supramolecule #1: Human immunodeficiency virus 1
| Supramolecule | Name: Human immunodeficiency virus 1 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: HIV-1 ADA.CM Env glycoprotein displayed on high Env expressing VLPs NCBI-ID: 11676 / Sci species name: Human immunodeficiency virus 1 / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / Strain: ADA.CM Homo sapiens (human) / Strain: ADA.CM |
| Molecular weight | Theoretical: 400 KDa |
| Virus shell | Shell ID: 1 / Name: Outer membrane envelope / Diameter: 1000.0 Å |
-Macromolecule #1: HIV Envelope glycoprotein
| Macromolecule | Name: HIV Envelope glycoprotein / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 / Strain: ADA.CM Human immunodeficiency virus 1 / Strain: ADA.CM |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARTEKLW VTVYYGVPVW KEATTTLFCA SDAKAYDTEV HNVWATHACV PTDPNPQEVV LENVTENFNM WKNNMVEQMH EDIISLWDQS LKPCVKLTPL CVTLNCTDLR NVTNSSSEGM RGEIKNCSFN ITTSIRDKVK ...String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARTEKLW VTVYYGVPVW KEATTTLFCA SDAKAYDTEV HNVWATHACV PTDPNPQEVV LENVTENFNM WKNNMVEQMH EDIISLWDQS LKPCVKLTPL CVTLNCTDLR NVTNSSSEGM RGEIKNCSFN ITTSIRDKVK KDYALFYRLD VVPIDNDNTS YRLINCNTST ITQACPKVSF EPIPIHYCTP AGFAILKCKD KKFNGTGPCK NVSTVQCTHG IRPVVSTQLL LNGSLAEEEV VIRSSNFTDN AKNIIVQLKE SVEINCTRPN NNTRKSIHIG PGRAFYTTGE IIGDIRQAHC NISRTKWNNT LNQIATKLKE QFGNNKTIVF NQSSGGDPEI VMHSFNCGGE FFYCNSTQLF NSTWNFNGTW NLTQSNGTEG NDTITLPCRI KQIINMWQEV GKAMYAPPIR GQIRCSSNIT GLILTRDGGT NSSGSEIFRP GGGDMRDNWR SELYKYKVVK IEPLGVAPTK AKRRVVQREK RAVGTIGAMF LGFLGAAGST MGAASMTLTV QARQLLSGIV QQQNNLLRAI EAQQHLLQLT VWGIRQLQAR VLAVERYLRD QQLLGIWGCS GKLICTTAVP WNASWSNKSL EQIWNNMTWM EWDREINNYT SLIHSLIEEA QNQQEKNEQE LLELDKWASL WNWFNITNWL WYIKLFIMIV GGLVGLRIVF AVLSIVNRVR QGYSPLSFQT HLPTPRGPDR PEGIEEEGGE RDRDRSIRLV NGFLAL |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Component - Name: Phosphate buffer saline / Details: 1X PBS at pH 7.4 |
|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 3ul sample, blotting time of 4-5 seconds. |
| Details | VLP particles displaying nearly full-length Env glycoprotein on membrane surface |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 2.5 µm / Nominal magnification: 58000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Correlation coefficient |
 Movie
Movie Controller
Controller


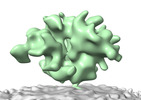














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)