[English] 日本語
 Yorodumi
Yorodumi- EMDB-24618: Archaeal DNA ligase and heterotrimeric PCNA in complex with non-l... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24618 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Archaeal DNA ligase and heterotrimeric PCNA in complex with non-ligatable DNA | |||||||||
 Map data Map data | Sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA ligase / PCNA / cryo-EM / LIGASE-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA ligase (ATP) / DNA ligase (ATP) activity / lagging strand elongation / DNA biosynthetic process / DNA polymerase processivity factor activity / leading strand elongation / regulation of DNA replication / DNA recombination / cell division / DNA repair ...DNA ligase (ATP) / DNA ligase (ATP) activity / lagging strand elongation / DNA biosynthetic process / DNA polymerase processivity factor activity / leading strand elongation / regulation of DNA replication / DNA recombination / cell division / DNA repair / DNA binding / ATP binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |   Saccharolobus solfataricus (archaea) / Saccharolobus solfataricus (archaea) /  Homo sapiens (human) Homo sapiens (human) | |||||||||
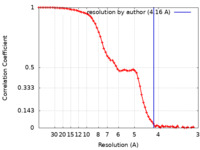
| Method | single particle reconstruction / cryo EM / Resolution: 4.16 Å | |||||||||
 Authors Authors | Sverzhinsky A / Pascal JM | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2022 Journal: Structure / Year: 2022Title: Cryo-EM structures and biochemical insights into heterotrimeric PCNA regulation of DNA ligase. Authors: Aleksandr Sverzhinsky / Alan E Tomkinson / John M Pascal /   Abstract: DNA ligases act in the final step of many DNA repair pathways and are commonly regulated by the DNA sliding clamp proliferating cell nuclear antigen (PCNA), but there are limited insights into the ...DNA ligases act in the final step of many DNA repair pathways and are commonly regulated by the DNA sliding clamp proliferating cell nuclear antigen (PCNA), but there are limited insights into the physical basis for this regulation. Here, we use single-particle cryoelectron microscopy (cryo-EM) to analyze an archaeal DNA ligase and heterotrimeric PCNA in complex with a single-strand DNA break. The cryo-EM structures highlight a continuous DNA-binding surface formed between DNA ligase and PCNA that supports the distorted conformation of the DNA break undergoing repair and contributes to PCNA stimulation of DNA ligation. DNA ligase is conformationally flexible within the complex, with its domains fully ordered only when encircling the repaired DNA to form a stacked ring structure with PCNA. The structures highlight DNA ligase structural transitions while docked on PCNA, changes in DNA conformation during ligation, and the potential for DNA ligase domains to regulate PCNA accessibility to other repair factors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24618.map.gz emd_24618.map.gz | 21.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24618-v30.xml emd-24618-v30.xml emd-24618.xml emd-24618.xml | 23.6 KB 23.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24618_fsc.xml emd_24618_fsc.xml | 8.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_24618.png emd_24618.png | 144.4 KB | ||
| Filedesc metadata |  emd-24618.cif.gz emd-24618.cif.gz | 7.2 KB | ||
| Others |  emd_24618_additional_1.map.gz emd_24618_additional_1.map.gz | 18.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24618 http://ftp.pdbj.org/pub/emdb/structures/EMD-24618 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24618 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24618 | HTTPS FTP |
-Related structure data
| Related structure data |  7rpoMC  7rpwC  7rpxC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24618.map.gz / Format: CCP4 / Size: 24.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24618.map.gz / Format: CCP4 / Size: 24.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: #1
| File | emd_24618_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Ternary complex of DNA Ligase with PCNA1-2-3 and non-ligatable DNA
+Supramolecule #1: Ternary complex of DNA Ligase with PCNA1-2-3 and non-ligatable DNA
+Macromolecule #1: DNA polymerase sliding clamp 1
+Macromolecule #2: DNA polymerase sliding clamp 2
+Macromolecule #3: DNA polymerase sliding clamp 3
+Macromolecule #7: DNA ligase
+Macromolecule #4: Upstream strand DNA
+Macromolecule #5: Downstream strand DNA
+Macromolecule #6: Template strand DNA
+Macromolecule #8: MANGANESE (II) ION
+Macromolecule #9: ADENOSINE MONOPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.175 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: wait time 0, blot force 1, blot time 1, drain time 0. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 3014 / Average electron dose: 100.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Protocol: RIGID BODY FIT | ||||||||||||
| Output model |  PDB-7rpo: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)