[English] 日本語
 Yorodumi
Yorodumi- EMDB-23246: CryoEM map of SARS-CoV-2 S protein in complex with Receptor Bindi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23246 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM map of SARS-CoV-2 S protein in complex with Receptor Binding Domain antibody DH1041 | |||||||||
 Map data Map data | cryoEM map of SARS-CoV-2 S protein in complex with RBD antibody DH1041 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RBD antibody / DH1041 / SARS / COVID-19 / SARS-CoV-2 2P S ectodomain / VIRAL PROTEIN / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / Attachment and Entry / entry receptor-mediated virion attachment to host cell / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / endocytosis involved in viral entry into host cell / receptor ligand activity / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / host cell plasma membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.42 Å | |||||||||
 Authors Authors | Manne K / Acharya P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Authors: Dapeng Li / Robert J Edwards / Kartik Manne / David R Martinez / Alexandra Schäfer / S Munir Alam / Kevin Wiehe / Xiaozhi Lu / Robert Parks / Laura L Sutherland / Thomas H Oguin / Charlene ...Authors: Dapeng Li / Robert J Edwards / Kartik Manne / David R Martinez / Alexandra Schäfer / S Munir Alam / Kevin Wiehe / Xiaozhi Lu / Robert Parks / Laura L Sutherland / Thomas H Oguin / Charlene McDanal / Lautaro G Perez / Katayoun Mansouri / Sophie M C Gobeil / Katarzyna Janowska / Victoria Stalls / Megan Kopp / Fangping Cai / Esther Lee / Andrew Foulger / Giovanna E Hernandez / Aja Sanzone / Kedamawit Tilahun / Chuancang Jiang / Longping V Tse / Kevin W Bock / Mahnaz Minai / Bianca M Nagata / Kenneth Cronin / Victoria Gee-Lai / Margaret Deyton / Maggie Barr / Tarra Von Holle / Andrew N Macintyre / Erica Stover / Jared Feldman / Blake M Hauser / Timothy M Caradonna / Trevor D Scobey / Wes Rountree / Yunfei Wang / M Anthony Moody / Derek W Cain / C Todd DeMarco / Thomas N Denny / Christopher W Woods / Elizabeth W Petzold / Aaron G Schmidt / I-Ting Teng / Tongqing Zhou / Peter D Kwong / John R Mascola / Barney S Graham / Ian N Moore / Robert Seder / Hanne Andersen / Mark G Lewis / David C Montefiori / Gregory D Sempowski / Ralph S Baric / Priyamvada Acharya / Barton F Haynes / Kevin O Saunders /  Abstract: SARS-CoV-2-neutralizing antibodies (NAbs) protect against COVID-19. A concern regarding SARS-CoV-2 antibodies is whether they mediate disease enhancement. Here, we isolated NAbs against the receptor- ...SARS-CoV-2-neutralizing antibodies (NAbs) protect against COVID-19. A concern regarding SARS-CoV-2 antibodies is whether they mediate disease enhancement. Here, we isolated NAbs against the receptor-binding domain (RBD) or the N-terminal domain (NTD) of SARS-CoV-2 spike from individuals with acute or convalescent SARS-CoV-2 or a history of SARS-CoV infection. Cryo-electron microscopy of RBD and NTD antibodies demonstrated function-specific modes of binding. Select RBD NAbs also demonstrated Fc receptor-γ (FcγR)-mediated enhancement of virus infection in vitro, while five non-neutralizing NTD antibodies mediated FcγR-independent in vitro infection enhancement. However, both types of infection-enhancing antibodies protected from SARS-CoV-2 replication in monkeys and mice. Three of 46 monkeys infused with enhancing antibodies had higher lung inflammation scores compared to controls. One monkey had alveolar edema and elevated bronchoalveolar lavage inflammatory cytokines. Thus, while in vitro antibody-enhanced infection does not necessarily herald enhanced infection in vivo, increased lung inflammation can rarely occur in SARS-CoV-2 antibody-infused macaques. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies Authors: Li D / Edwards RJ / Manne K / Martinez DR / Schafer A / Alam SM / Wiehe K / Lu X / Parks R / Sutherland LL / Oguin TH / McDanal C / Perez LG / Mansouri K / Gobeil SMC / Janowska K / Stalls V ...Authors: Li D / Edwards RJ / Manne K / Martinez DR / Schafer A / Alam SM / Wiehe K / Lu X / Parks R / Sutherland LL / Oguin TH / McDanal C / Perez LG / Mansouri K / Gobeil SMC / Janowska K / Stalls V / Kopp M / Cai F / Lee E / Foulger A / Hernandez GE / Sanzone A / Tilahun K / Jiang C / Tse LV / Bock KW / Minai M / Nagata BM / Cronin K / Gee-Lai V / Deyton M / Barr M / Von Holle T / Macintyre AN / Stover E / Feldman J / Hauser BM / Caradonna TM / Scobey TD / Moody MA / Cain DW / DeMarco CT / Denny TN / Woods CW / Petzold EW / Schmidt AG / Teng IT / Zhou T / Kwong PD / Mascola JR / Graham BS / Moore IN / Seder R / Andersen H / Lewis MG / Montefiori DC / Sempowski GD / Baric RS / Acharya P / Haynes BF / Saunders KO | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23246.map.gz emd_23246.map.gz | 154.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23246-v30.xml emd-23246-v30.xml emd-23246.xml emd-23246.xml | 27.8 KB 27.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23246.png emd_23246.png | 38.5 KB | ||
| Filedesc metadata |  emd-23246.cif.gz emd-23246.cif.gz | 8 KB | ||
| Others |  emd_23246_half_map_1.map.gz emd_23246_half_map_1.map.gz emd_23246_half_map_2.map.gz emd_23246_half_map_2.map.gz | 151.9 MB 151.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23246 http://ftp.pdbj.org/pub/emdb/structures/EMD-23246 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23246 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23246 | HTTPS FTP |
-Related structure data
| Related structure data |  7laaMC  7labC  7lcnC  7ld1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23246.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23246.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryoEM map of SARS-CoV-2 S protein in complex with RBD antibody DH1041 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.058 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: cryoEM half-map A of SARS-CoV-2 S protein in...
| File | emd_23246_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryoEM half-map A of SARS-CoV-2 S protein in complex with RBD antibody DH1041 | ||||||||||||
| Projections & Slices |
| ||||||||||||
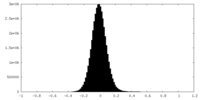
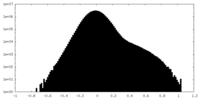
| Density Histograms |
-Half map: cryoEM half-map B of SARS-CoV-2 S protein in...
| File | emd_23246_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryoEM half-map B of SARS-CoV-2 S protein in complex with RBD antibody DH1041 | ||||||||||||
| Projections & Slices |
| ||||||||||||
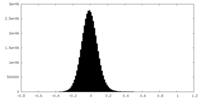
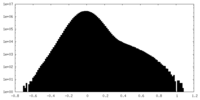
| Density Histograms |
- Sample components
Sample components
-Entire : Severe acute respiratory syndrome-related coronavirus spike glyco...
| Entire | Name: Severe acute respiratory syndrome-related coronavirus spike glycoprotein in complex with RBD antibody DH1041 |
|---|---|
| Components |
|
-Supramolecule #1: Severe acute respiratory syndrome-related coronavirus spike glyco...
| Supramolecule | Name: Severe acute respiratory syndrome-related coronavirus spike glycoprotein in complex with RBD antibody DH1041 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: Spike glycoprotein
| Supramolecule | Name: Spike glycoprotein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Antibody DH1041
| Supramolecule | Name: Antibody DH1041 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 124.125477 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AYTNSFTRGV YYPDKVFRSS VLHSTQDLFL PFFSNVTWFH AIHVSGTNGT KRFDNPVLPF NDGVYFASTE KSNIIRGWIF GTTLDSKTQ SLLIVNNATN VVIKVCEFQF CNDPFLGVYY HKNNKSWMES EFRVYSSANN CTFEYVSQPF LMDLEGKQGN F KNLREFVF ...String: AYTNSFTRGV YYPDKVFRSS VLHSTQDLFL PFFSNVTWFH AIHVSGTNGT KRFDNPVLPF NDGVYFASTE KSNIIRGWIF GTTLDSKTQ SLLIVNNATN VVIKVCEFQF CNDPFLGVYY HKNNKSWMES EFRVYSSANN CTFEYVSQPF LMDLEGKQGN F KNLREFVF KNIDGYFKIY SKHTPINLVR DLPQGFSALE PLVDLPIGIN ITRFQTLLAL HRSYLTPGDS SSGWTAGAAA YY VGYLQPR TFLLKYNENG TITDAVDCAL DPLSETKCTL KSFTVEKGIY QTSNFRVQPT ESIVRFPNIT NLCPFGEVFN ATR FASVYA WNRKRISNCV ADYSVLYNSA SFSTFKCYGV SPTKLNDLCF TNVYADSFVI RGDEVRQIAP GQTGKIADYN YKLP DDFTG CVIAWNSNNL DSKVGGNYNY LYRLFRKSNL KPFERDISTE IYQAGSTPCN GVEGFNCYFP LQSYGFQPTN GVGYQ PYRV VVLSFELLHA PATVCGPKKS TNLVKNKCVN FNFNGLTGTG VLTESNKKFL PFQQFGRDIA DTTDAVRDPQ TLEILD ITP CSFGGVSVIT PGTNTSNEVA VLYQDVNCTE VPVAIHADQL TPTWRVYSTG SNVFQTRAGC LIGAEHVNNS YECDIPI GA GICASYQTQT NSPRRARSVA SQSIIAYTMS LGAENSVAYS NNSIAIPTNF TISVTTEILP VSMTKTSVDC TMYICGDS T ECSNLLLQYG SFCTQLNRAL TGIAVEQDKN TQEVFAQVKQ IYKTPPIKDF GGFNFSQILP DPSKPSKRSF IEDLLFNKV TLADAGFIKQ YGDCLGDIAA RDLICAQKFN GLTVLPPLLT DEMIAQYTSA LLAGTITSGW TFGAGAALQI PFAMQMAYRF NGIGVTQNV LYENQKLIAN QFNSAIGKIQ DSLSSTASAL GKLQDVVNQN AQALNTLVKQ LSSNFGAISS VLNDILSRLD P PEAEVQID RLITGRLQSL QTYVTQQLIR AAEIRASANL AATKMSECVL GQSKRVDFCG KGYHLMSFPQ SAPHGVVFLH VT YVPAQEK NFTTAPAICH DGKAHFPREG VFVSNGTHWF VTQRNFYEPQ IITTDNTFVS GNCDVVIGIV NNTVYDPLQP ELD S UniProtKB: Spike glycoprotein |
-Macromolecule #2: DH1041 heavy chain
| Macromolecule | Name: DH1041 heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.125973 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLVESGGG LVQPGGSLRL SCAASGVTFS SYWMSWVRQA PGKGLEWVAN IRQDGSEKYS VDSVKGRFTI SRDNAKNSLY LQMNSLRAE DTAVYYCARA RRADNSGYYG FHFDCWGQGT LVTVSSASTK GPSVFPLAPS SKSTSGGTAA LGCLVKDYFP E PVTVSWNS ...String: EVQLVESGGG LVQPGGSLRL SCAASGVTFS SYWMSWVRQA PGKGLEWVAN IRQDGSEKYS VDSVKGRFTI SRDNAKNSLY LQMNSLRAE DTAVYYCARA RRADNSGYYG FHFDCWGQGT LVTVSSASTK GPSVFPLAPS SKSTSGGTAA LGCLVKDYFP E PVTVSWNS GALTSGVHTF PAVLQSSGLY SLSSVVTVPS SSLGTQTYIC NVNHKPSNTK VDKKVEPK |
-Macromolecule #3: DH1041 light chain
| Macromolecule | Name: DH1041 light chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.043367 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVLTQPPSV SGAPGQRVTI SCTGSSSNIG AGYDVHWYQQ LPGTAPKLLI YGNNNRPSGV PDRFSDSKSG TSASLAITRL QAEDEADYY CQSYDSSLSG WVFGGGTKLT VLGQPKANPT VTLFPPSSEE LQANKATLVC LISDFYPGAV TVAWKADSSP V KAGVETTT ...String: QSVLTQPPSV SGAPGQRVTI SCTGSSSNIG AGYDVHWYQQ LPGTAPKLLI YGNNNRPSGV PDRFSDSKSG TSASLAITRL QAEDEADYY CQSYDSSLSG WVFGGGTKLT VLGQPKANPT VTLFPPSSEE LQANKATLVC LISDFYPGAV TVAWKADSSP V KAGVETTT PSKQSNNKYA ASSYLSLTPE QWKSHRSYSC QVTHEGSTVE KTVAPTECS |
-Macromolecule #6: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 6 / Number of copies: 17 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 93.15 K / Max: 93.15 K |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 65.94 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)