[English] 日本語
 Yorodumi
Yorodumi- EMDB-2203: Characterization of the insertase for beta-barrel proteins of the... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2203 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Characterization of the insertase for beta-barrel proteins of the outer mitochondrial membrane. 3-D reconstruction of the TOB complex | |||||||||
 Map data Map data | 3D reconstruction of Tob55 dimers isolated using the 9xHis tag on the Tob55 subunit. The Tob55 dimer is a complex identified in all outer membrane preparations but isolated only when the his-tag is on tob55. The dimer co-purifies with the TOB complex and was identified as a subclass of particles present in the his-Tob55 data sets. The mol. wgt. is 101 kDa. Two-fold symmetry has been applied | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TOB/SAM complex / beta-barrel proteins / Tob55/Sam50 / mitochondria outer membrane | |||||||||
| Biological species |  Neurospora crassa (fungus) Neurospora crassa (fungus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 13.4 Å | |||||||||
 Authors Authors | Klein A / Israel L / Lackey SWK / Nargang FE / Imhof A / Baumeister W / Neupert W / Thomas DR | |||||||||
 Citation Citation |  Journal: J Cell Biol / Year: 2012 Journal: J Cell Biol / Year: 2012Title: Characterization of the insertase for β-barrel proteins of the outer mitochondrial membrane. Authors: Astrid Klein / Lars Israel / Sebastian W K Lackey / Frank E Nargang / Axel Imhof / Wolfgang Baumeister / Walter Neupert / Dennis R Thomas /  Abstract: The TOB-SAM complex is an essential component of the mitochondrial outer membrane that mediates the insertion of β-barrel precursor proteins into the membrane. We report here its isolation and ...The TOB-SAM complex is an essential component of the mitochondrial outer membrane that mediates the insertion of β-barrel precursor proteins into the membrane. We report here its isolation and determine its size, composition, and structural organization. The complex from Neurospora crassa was composed of Tob55-Sam50, Tob38-Sam35, and Tob37-Sam37 in a stoichiometry of 1:1:1 and had a molecular mass of 140 kD. A very minor fraction of the purified complex was associated with one Mdm10 protein. Using molecular homology modeling for Tob55 and cryoelectron microscopy reconstructions of the TOB complex, we present a model of the TOB-SAM complex that integrates biochemical and structural data. We discuss our results and the structural model in the context of a possible mechanism of the TOB insertase. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2203.map.gz emd_2203.map.gz | 9.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2203-v30.xml emd-2203-v30.xml emd-2203.xml emd-2203.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2203.jpg EMD-2203.jpg | 29.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2203 http://ftp.pdbj.org/pub/emdb/structures/EMD-2203 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2203 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2203 | HTTPS FTP |
-Related structure data
| Related structure data |  2195C 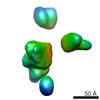 2196C  2197C  2200C  2201C  2202C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2203.map.gz / Format: CCP4 / Size: 10.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2203.map.gz / Format: CCP4 / Size: 10.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D reconstruction of Tob55 dimers isolated using the 9xHis tag on the Tob55 subunit. The Tob55 dimer is a complex identified in all outer membrane preparations but isolated only when the his-tag is on tob55. The dimer co-purifies with the TOB complex and was identified as a subclass of particles present in the his-Tob55 data sets. The mol. wgt. is 101 kDa. Two-fold symmetry has been applied | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.78 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Tob55 dimer
| Entire | Name: Tob55 dimer |
|---|---|
| Components |
|
-Supramolecule #1000: Tob55 dimer
| Supramolecule | Name: Tob55 dimer / type: sample / ID: 1000 Details: The complexes were monodisperse. A mitochondrial outer membrane complex which can be identified as a component of the outer mitochondrial membrane but whose function is unknown. Oligomeric state: dimeric / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 101 KDa / Theoretical: 101 KDa Method: Blue native gel electrophoresis and Isotope dilution mass spectroscopy analysis of bands isolated from BNGE gels. |
-Macromolecule #1: Tob55 short
| Macromolecule | Name: Tob55 short / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Oligomeric state: dimer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) / Strain: Tob55 short HT / Organelle: mitochondria / Location in cell: outer membrane Neurospora crassa (fungus) / Strain: Tob55 short HT / Organelle: mitochondria / Location in cell: outer membrane |
| Molecular weight | Experimental: 50.7 KDa / Theoretical: 50.7 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 8.5 Details: 1mM PMSF, 0.08%(v/v) Triton X-100, 50 mM HEPES pH 8.5 |
| Grid | Details: lacey carbon films on 200 mesh Molybdenum grids |
| Vitrification | Cryogen name: ETHANE / Instrument: HOMEMADE PLUNGER Method: blot for 4-5 seconds before plunging with whatman filter paper #1 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected using the live FFT at imaging magnification. |
| Date | Oct 7, 2009 |
| Image recording | Category: CCD / Film or detector model: FEI EAGLE (4k x 4k) / Average electron dose: 20 e/Å2 Details: Images collected using TOM_acquisition software. 24800 dimers were identified in various his-Tob55 datasets. In the end 19500 were included in the final reconstruction. Two-fold symmetry was ...Details: Images collected using TOM_acquisition software. 24800 dimers were identified in various his-Tob55 datasets. In the end 19500 were included in the final reconstruction. Two-fold symmetry was applied during the last 5 rounds of refinement. |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 84270 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: -3.7 µm / Nominal defocus min: -0.5 µm / Nominal magnification: 62000 |
| Sample stage | Specimen holder: Gatan 656 side entry holder / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: Phase and astigmatism correction applied to each micrograph |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 13.4 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPIDER, TOM_toolbox Details: Final maps were reconstructed from images that had stable alignment parameters over the last 4 rounds of refinement. Stable was defined by absolute accumulated changes in theta and psi of ...Details: Final maps were reconstructed from images that had stable alignment parameters over the last 4 rounds of refinement. Stable was defined by absolute accumulated changes in theta and psi of the projection matched of less than 10 degrees. 24800 dimers were identified in various his-Tob55 datasets. In the end 19500 were included in the final reconstruction. Two-fold symmetry was applied during the last 5 rounds of refinement. Number images used: 1 |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)