+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20609 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
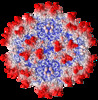
| Title | Cryo-EM structure of the chimeric vector AAV2.7m8 | |||||||||
 Map data Map data | chimeric vector AAV2.7m8 | |||||||||
 Sample Sample | Adeno-associated virus - 2.7m8 != Adeno-associated virus - 2 Adeno-associated virus - 2.7m8
| |||||||||
 Keywords Keywords | Protrusion / insertion / receptor / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont entry into host cell via permeabilization of host membrane / host cell nucleolus / T=1 icosahedral viral capsid / clathrin-dependent endocytosis of virus by host cell / virion attachment to host cell / structural molecule activity Similarity search - Function | |||||||||
| Biological species |  Adeno-associated virus - 2 Adeno-associated virus - 2 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.91 Å | |||||||||
 Authors Authors | Agbandje-McKenna M / Bennett A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2020 Journal: J Struct Biol / Year: 2020Title: Structure comparison of the chimeric AAV2.7m8 vector with parental AAV2. Authors: Antonette Bennett / Annahita Keravala / Victoria Makal / Justin Kurian / Brahim Belbellaa / Rangoli Aeran / Yu-Shan Tseng / Duncan Sousa / John Spear / Mehdi Gasmi / Mavis Agbandje-McKenna /  Abstract: The AAV2.7m8 vector is an engineered capsid with a 10-amino acid insertion in adeno-associated virus (AAV) surface variable region VIII (VR-VIII) resulting in the alteration of an antigenic region of ...The AAV2.7m8 vector is an engineered capsid with a 10-amino acid insertion in adeno-associated virus (AAV) surface variable region VIII (VR-VIII) resulting in the alteration of an antigenic region of AAV2 and the ability to efficiently transduce retina cells following intravitreal administration. Directed evolution and in vivo screening in the mouse retina isolated this vector. In the present study, we sought to identify the structural differences between a recombinant AAV2.7m8 (rAAV2.7m8) vector packaging a GFP genome and its parental serotype, AAV2, by cryo-electron microscopy (cryo-EM) and image reconstruction. The structures of rAAV2.7m8 and AAV2 were determined to 2.91 and 3.02 Å resolution, respectively. The rAAV2.7m8 amino acid side-chains for residues 219-745 (the last C-terminal residue) were interpretable in the density map with the exception of the 10 inserted amino acids. While observable in a low sigma threshold density, side-chains were only resolved at the base of the insertion, likely due to flexibility at the top of the loop. A comparison to parental AAV2 (ordered from residues 217-735) showed the structures to be similar, except at some side-chains that had different orientations and, in VR-VIII containing the 10 amino acid insertion. VR-VIII is part of an AAV2 antigenic epitope, and the difference is consistent with rAAV2.7m8's escape from a known AAV2 monoclonal antibody, C37-B. The observations provide valuable insight into the configuration of inserted surface peptides on the AAV capsid and structural differences to be leveraged for future AAV vector rational design, especially for retargeted tropism and antibody escape. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20609.map.gz emd_20609.map.gz | 94.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20609-v30.xml emd-20609-v30.xml emd-20609.xml emd-20609.xml | 10.6 KB 10.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20609.png emd_20609.png | 293.2 KB | ||
| Filedesc metadata |  emd-20609.cif.gz emd-20609.cif.gz | 5.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20609 http://ftp.pdbj.org/pub/emdb/structures/EMD-20609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20609 | HTTPS FTP |
-Related structure data
| Related structure data |  6u0rMC  6u0vC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20609.map.gz / Format: CCP4 / Size: 284.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20609.map.gz / Format: CCP4 / Size: 284.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | chimeric vector AAV2.7m8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Adeno-associated virus - 2.7m8
| Entire | Name: Adeno-associated virus - 2.7m8 |
|---|---|
| Components |
|
-Supramolecule #1: Adeno-associated virus - 2
| Supramolecule | Name: Adeno-associated virus - 2 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1 / NCBI-ID: 10804 / Sci species name: Adeno-associated virus - 2 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Molecular weight | Theoretical: 4 MDa |
| Virus shell | Shell ID: 1 / Diameter: 260.0 Å / T number (triangulation number): 1 |
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 60 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Adeno-associated virus - 2 Adeno-associated virus - 2 |
| Molecular weight | Theoretical: 59.655652 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DGVGNSSGNW HCDSTWMGDR VITTSTRTWA LPTYNNHLYK QISSQSGASN DNHYFGYSTP WGYFDFNRFH CHFSPRDWQR LINNNWGFR PKRLNFKLFN IQVKEVTQND GTTTIANNLT STVQVFTDSE YQLPYVLGSA HQGCLPPFPA DVFMVPQYGY L TLNNGSQA ...String: DGVGNSSGNW HCDSTWMGDR VITTSTRTWA LPTYNNHLYK QISSQSGASN DNHYFGYSTP WGYFDFNRFH CHFSPRDWQR LINNNWGFR PKRLNFKLFN IQVKEVTQND GTTTIANNLT STVQVFTDSE YQLPYVLGSA HQGCLPPFPA DVFMVPQYGY L TLNNGSQA VGRSSFYCLE YFPSQMLRTG NNFTFSYTFE DVPFHSSYAH SQSLDRLMNP LIDQYLYYLS RTNTPSGTTT QS RLQFSQA GASDIRDQSR NWLPGPCYRQ QRVSKTSADN NNSEYSWTGA TKYHLNGRDS LVNPGPAMAS HKDDEEKFFP QSG VLIFGK QGSEKTNVDI EKVMITDEEE IRTTNPVATE QYGSVSTNLQ RGNLALGETT RPARQAATAD VNTQGVLPGM VWQD RDVYL QGPIWAKIPH TDGHFHPSPL MGGFGLKHPP PQILIKNTPV PANPSTTFSA AKFASFITQY STGQVSVEIE WELQK ENSK RWNPEIQYTS NYNKSVNVDF TVDTNGVYSE PRPIGTRYLT RNL UniProtKB: Capsid protein VP1 |
-Macromolecule #2: 2'-DEOXYADENOSINE-5'-MONOPHOSPHATE
| Macromolecule | Name: 2'-DEOXYADENOSINE-5'-MONOPHOSPHATE / type: ligand / ID: 2 / Number of copies: 60 / Formula: D5M |
|---|---|
| Molecular weight | Theoretical: 331.222 Da |
| Chemical component information | 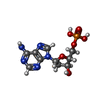 ChemComp-D5M: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 410 / Average electron dose: 75.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT / Overall B value: 100 |
|---|---|
| Output model |  PDB-6u0r: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)





















