+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20182 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Dimeric DNA complex | |||||||||
 Map data Map data | dimeric DNA complex | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
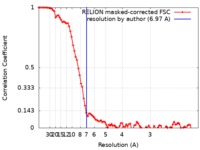
| Method | single particle reconstruction / cryo EM / Resolution: 6.97 Å | |||||||||
 Authors Authors | Chang L / Li Z / Zhang H | |||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2019 Journal: Cell Host Microbe / Year: 2019Title: Structural Basis for the Inhibition of CRISPR-Cas12a by Anti-CRISPR Proteins. Authors: Heng Zhang / Zhuang Li / Courtney M Daczkowski / Clinton Gabel / Andrew D Mesecar / Leifu Chang /  Abstract: CRISPR-Cas12a (Cpf1), a type V CRISPR-associated nuclease, provides bacterial immunity against bacteriophages and plasmids but also serves as a tool for genome editing. Foreign nucleic acids are ...CRISPR-Cas12a (Cpf1), a type V CRISPR-associated nuclease, provides bacterial immunity against bacteriophages and plasmids but also serves as a tool for genome editing. Foreign nucleic acids are integrated into the CRISPR locus, prompting transcription of CRISPR RNAs (crRNAs) that guide Cas12a cleavage of foreign complementary DNA. However, mobile genetic elements counteract Cas12a with inhibitors, notably type V-A anti-CRISPRs (AcrVAs). We present cryoelectron microscopy structures of Cas12a-crRNA bound to AcrVA1 and AcrVA4 at 3.5 and 3.3 Å resolutions, respectively. AcrVA1 is sandwiched between the recognition (REC) and nuclease (NUC) lobes of Cas12a and inserts into the binding pocket for the protospacer-adjacent motif (PAM), a short DNA sequence guiding Cas12a targeting. AcrVA1 cleaves crRNA in a Cas12a-dependent manner, inactivating Cas12a-crRNA complexes. The AcrVA4 dimer is anchored around the crRNA pseudoknot of Cas12a-crRNA, preventing required conformational changes for crRNA-DNA heteroduplex formation. These results uncover molecular mechanisms for CRISPR-Cas12a inhibition, providing insights into bacteria-phage dynamics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20182.map.gz emd_20182.map.gz | 5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20182-v30.xml emd-20182-v30.xml emd-20182.xml emd-20182.xml | 7.3 KB 7.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20182_fsc.xml emd_20182_fsc.xml | 8.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_20182.png emd_20182.png | 61.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20182 http://ftp.pdbj.org/pub/emdb/structures/EMD-20182 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20182 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20182 | HTTPS FTP |
-Related structure data
| Related structure data |  0445C  0446C  0447C  0449C  9398C  6nm9C  6nmaC  6nmcC  6nmdC  6nmeC  6omvC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_20182.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20182.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | dimeric DNA complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : dimeric DNA complex
| Entire | Name: dimeric DNA complex |
|---|---|
| Components |
|
-Supramolecule #1: dimeric DNA complex
| Supramolecule | Name: dimeric DNA complex / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)