+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | MYC-MAX bound to a nucleosome at SHL+5.8 | |||||||||
 Map data Map data | Map post-processed with LocScale. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E-box / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationMad-Max complex / Deposition of new CENPA-containing nucleosomes at the centromere / Inhibition of DNA recombination at telomere / positive regulation of metanephric cap mesenchymal cell proliferation / positive regulation of acinar cell proliferation / acinar cell proliferation / negative regulation of transcription initiation by RNA polymerase II / SCF ubiquitin ligase complex binding / NK T cell proliferation / Myc-Max complex ...Mad-Max complex / Deposition of new CENPA-containing nucleosomes at the centromere / Inhibition of DNA recombination at telomere / positive regulation of metanephric cap mesenchymal cell proliferation / positive regulation of acinar cell proliferation / acinar cell proliferation / negative regulation of transcription initiation by RNA polymerase II / SCF ubiquitin ligase complex binding / NK T cell proliferation / Myc-Max complex / DNA Damage/Telomere Stress Induced Senescence / Regulation of endogenous retroelements by KRAB-ZFP proteins / Condensation of Prophase Chromosomes / Recognition and association of DNA glycosylase with site containing an affected purine / Metalloprotease DUBs / Cleavage of the damaged purine / regulation of somatic stem cell population maintenance / HDACs deacetylate histones / regulation of cell cycle process / PRC2 methylates histones and DNA / UCH proteinases / RNA polymerase II transcription repressor complex / cellular response to interferon-alpha / Binding of TCF/LEF:CTNNB1 to target gene promoters / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / positive regulation of B cell apoptotic process / RUNX3 regulates WNT signaling / TFAP2 (AP-2) family regulates transcription of cell cycle factors / myotube differentiation / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / RMTs methylate histone arginines / negative regulation of cell division / negative regulation of monocyte differentiation / detection of mechanical stimulus involved in sensory perception of sound / response to growth factor / Ub-specific processing proteases / response to alkaloid / B cell apoptotic process / transcription regulator activator activity / Transcription of E2F targets under negative control by DREAM complex / fibroblast apoptotic process / negative regulation of stress-activated MAPK cascade / Regulation of NFE2L2 gene expression / protein-DNA complex disassembly / positive regulation of mesenchymal cell proliferation / regulation of telomere maintenance / skeletal system morphogenesis / Signaling by ALK / branching involved in ureteric bud morphogenesis / middle ear morphogenesis / rRNA metabolic process / negative regulation of gene expression via chromosomal CpG island methylation / Transcriptional Regulation by E2F6 / pigmentation / E-box binding / positive regulation of telomere maintenance / MLL1 complex / skeletal muscle cell differentiation / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / chromosome organization / positive regulation of transcription initiation by RNA polymerase II / negative regulation of tumor necrosis factor-mediated signaling pathway / core promoter sequence-specific DNA binding / Cyclin E associated events during G1/S transition / Cyclin A:Cdk2-associated events at S phase entry / negative regulation of fibroblast proliferation / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / ERK1 and ERK2 cascade / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / epigenetic regulation of gene expression / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / positive regulation of epithelial cell proliferation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / transcription coregulator binding / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / innate immune response in mucosa / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Negative Regulation of CDH1 Gene Transcription Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Stoos L / Michael AK / Kempf G / Kater L / Cavadini S / Thoma N | |||||||||
| Funding support | European Union,  France, 2 items France, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Cooperation between bHLH transcription factors and histones for DNA access. Authors: Alicia K Michael / Lisa Stoos / Priya Crosby / Nikolas Eggers / Xinyu Y Nie / Kristina Makasheva / Martina Minnich / Kelly L Healy / Joscha Weiss / Georg Kempf / Simone Cavadini / Lukas ...Authors: Alicia K Michael / Lisa Stoos / Priya Crosby / Nikolas Eggers / Xinyu Y Nie / Kristina Makasheva / Martina Minnich / Kelly L Healy / Joscha Weiss / Georg Kempf / Simone Cavadini / Lukas Kater / Jan Seebacher / Luca Vecchia / Deyasini Chakraborty / Luke Isbel / Ralph S Grand / Florian Andersch / Jennifer L Fribourgh / Dirk Schübeler / Johannes Zuber / Andrew C Liu / Peter B Becker / Beat Fierz / Carrie L Partch / Jerome S Menet / Nicolas H Thomä /     Abstract: The basic helix-loop-helix (bHLH) family of transcription factors recognizes DNA motifs known as E-boxes (CANNTG) and includes 108 members. Here we investigate how chromatinized E-boxes are engaged ...The basic helix-loop-helix (bHLH) family of transcription factors recognizes DNA motifs known as E-boxes (CANNTG) and includes 108 members. Here we investigate how chromatinized E-boxes are engaged by two structurally diverse bHLH proteins: the proto-oncogene MYC-MAX and the circadian transcription factor CLOCK-BMAL1 (refs. ). Both transcription factors bind to E-boxes preferentially near the nucleosomal entry-exit sites. Structural studies with engineered or native nucleosome sequences show that MYC-MAX or CLOCK-BMAL1 triggers the release of DNA from histones to gain access. Atop the H2A-H2B acidic patch, the CLOCK-BMAL1 Per-Arnt-Sim (PAS) dimerization domains engage the histone octamer disc. Binding of tandem E-boxes at endogenous DNA sequences occurs through direct interactions between two CLOCK-BMAL1 protomers and histones and is important for circadian cycling. At internal E-boxes, the MYC-MAX leucine zipper can also interact with histones H2B and H3, and its binding is indirectly enhanced by OCT4 elsewhere on the nucleosome. The nucleosomal E-box position and the type of bHLH dimerization domain jointly determine the histone contact, the affinity and the degree of competition and cooperativity with other nucleosome-bound factors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17184.map.gz emd_17184.map.gz | 61.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17184-v30.xml emd-17184-v30.xml emd-17184.xml emd-17184.xml | 29.3 KB 29.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17184_fsc.xml emd_17184_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_17184.png emd_17184.png | 110.6 KB | ||
| Filedesc metadata |  emd-17184.cif.gz emd-17184.cif.gz | 7 KB | ||
| Others |  emd_17184_additional_1.map.gz emd_17184_additional_1.map.gz emd_17184_half_map_1.map.gz emd_17184_half_map_1.map.gz emd_17184_half_map_2.map.gz emd_17184_half_map_2.map.gz | 62.2 MB 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17184 http://ftp.pdbj.org/pub/emdb/structures/EMD-17184 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17184 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17184 | HTTPS FTP |
-Related structure data
| Related structure data |  8ottMC  8osjC  8oskC  8oslC  8otsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17184.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17184.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map post-processed with LocScale. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.845 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Full map.
| File | emd_17184_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map A.
| File | emd_17184_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map B.
| File | emd_17184_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map B. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : MYC-MAX bound to a nucleosome at SHL+5.8
+Supramolecule #1: MYC-MAX bound to a nucleosome at SHL+5.8
+Supramolecule #2: Nucleosomal core particle
+Supramolecule #3: cMYC/MAX heterodimer
+Supramolecule #4: Histone octamer
+Supramolecule #5: Nucleosomal DNA
+Macromolecule #1: Histone H3.1
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A type 1-B/E
+Macromolecule #4: Histone H2B type 1-J
+Macromolecule #5: Histone H2A type 1-K
+Macromolecule #8: Myc proto-oncogene protein
+Macromolecule #9: Protein max
+Macromolecule #6: DNA (144-MER)
+Macromolecule #7: DNA (144-MER)
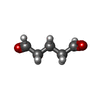
+Macromolecule #10: PENTANEDIAL
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)