[English] 日本語
 Yorodumi
Yorodumi- EMDB-17160: Cryo-EM structure of CLOCK-BMAL1 bound to the native Por enhancer... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of CLOCK-BMAL1 bound to the native Por enhancer nucleosome (map 2, additional 3D classification and flexible refinement) | |||||||||
 Map data Map data | Full-map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E-box / transcription factor / circadian clock / GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationCLOCK-BMAL transcription complex / positive regulation of skeletal muscle cell differentiation / regulation of hair cycle / positive regulation of protein acetylation / NPAS4 regulates expression of target genes / negative regulation of nuclear receptor-mediated glucocorticoid signaling pathway / maternal process involved in parturition / regulation of type B pancreatic cell development / bHLH transcription factor binding / regulation of cellular senescence ...CLOCK-BMAL transcription complex / positive regulation of skeletal muscle cell differentiation / regulation of hair cycle / positive regulation of protein acetylation / NPAS4 regulates expression of target genes / negative regulation of nuclear receptor-mediated glucocorticoid signaling pathway / maternal process involved in parturition / regulation of type B pancreatic cell development / bHLH transcription factor binding / regulation of cellular senescence / chromatoid body / positive regulation of circadian rhythm / oxidative stress-induced premature senescence / negative regulation of TOR signaling / negative regulation of cold-induced thermogenesis / response to redox state / protein acetylation / negative regulation of fat cell differentiation / regulation of protein catabolic process / E-box binding / histone acetyltransferase activity / regulation of neurogenesis / negative regulation of tumor necrosis factor-mediated signaling pathway / histone acetyltransferase / energy homeostasis / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / regulation of insulin secretion / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / epigenetic regulation of gene expression / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / DNA damage checkpoint signaling / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / cellular response to ionizing radiation / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / innate immune response in mucosa / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / transcription coregulator activity / Negative Regulation of CDH1 Gene Transcription / circadian regulation of gene expression / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / Nonhomologous End-Joining (NHEJ) / RNA Polymerase I Promoter Escape / lipopolysaccharide binding / Transcriptional regulation by small RNAs / circadian rhythm / Formation of the beta-catenin:TCF transactivating complex / : / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / HDMs demethylate histones / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / regulation of circadian rhythm / PML body / chromatin DNA binding / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / autophagy / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / Transcriptional regulation of granulopoiesis / RMTs methylate histone arginines / protein import into nucleus / HCMV Early Events / positive regulation of inflammatory response / structural constituent of chromatin / UCH proteinases / positive regulation of canonical Wnt signaling pathway / heterochromatin formation / nucleosome / antimicrobial humoral immune response mediated by antimicrobial peptide / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / antibacterial humoral response Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) / Homo sapiens (human) / synthetic construct (others) /  | |||||||||
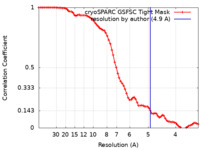
| Method | single particle reconstruction / cryo EM / Resolution: 4.9 Å | |||||||||
 Authors Authors | Michael AK / Stoos L / Kempf G / Cavadini S / Thoma N | |||||||||
| Funding support | European Union,  France, 2 items France, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Cooperation between bHLH transcription factors and histones for DNA access. Authors: Alicia K Michael / Lisa Stoos / Priya Crosby / Nikolas Eggers / Xinyu Y Nie / Kristina Makasheva / Martina Minnich / Kelly L Healy / Joscha Weiss / Georg Kempf / Simone Cavadini / Lukas ...Authors: Alicia K Michael / Lisa Stoos / Priya Crosby / Nikolas Eggers / Xinyu Y Nie / Kristina Makasheva / Martina Minnich / Kelly L Healy / Joscha Weiss / Georg Kempf / Simone Cavadini / Lukas Kater / Jan Seebacher / Luca Vecchia / Deyasini Chakraborty / Luke Isbel / Ralph S Grand / Florian Andersch / Jennifer L Fribourgh / Dirk Schübeler / Johannes Zuber / Andrew C Liu / Peter B Becker / Beat Fierz / Carrie L Partch / Jerome S Menet / Nicolas H Thomä /     Abstract: The basic helix-loop-helix (bHLH) family of transcription factors recognizes DNA motifs known as E-boxes (CANNTG) and includes 108 members. Here we investigate how chromatinized E-boxes are engaged ...The basic helix-loop-helix (bHLH) family of transcription factors recognizes DNA motifs known as E-boxes (CANNTG) and includes 108 members. Here we investigate how chromatinized E-boxes are engaged by two structurally diverse bHLH proteins: the proto-oncogene MYC-MAX and the circadian transcription factor CLOCK-BMAL1 (refs. ). Both transcription factors bind to E-boxes preferentially near the nucleosomal entry-exit sites. Structural studies with engineered or native nucleosome sequences show that MYC-MAX or CLOCK-BMAL1 triggers the release of DNA from histones to gain access. Atop the H2A-H2B acidic patch, the CLOCK-BMAL1 Per-Arnt-Sim (PAS) dimerization domains engage the histone octamer disc. Binding of tandem E-boxes at endogenous DNA sequences occurs through direct interactions between two CLOCK-BMAL1 protomers and histones and is important for circadian cycling. At internal E-boxes, the MYC-MAX leucine zipper can also interact with histones H2B and H3, and its binding is indirectly enhanced by OCT4 elsewhere on the nucleosome. The nucleosomal E-box position and the type of bHLH dimerization domain jointly determine the histone contact, the affinity and the degree of competition and cooperativity with other nucleosome-bound factors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17160.map.gz emd_17160.map.gz | 19.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17160-v30.xml emd-17160-v30.xml emd-17160.xml emd-17160.xml | 29.5 KB 29.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17160_fsc.xml emd_17160_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_17160.png emd_17160.png | 75.5 KB | ||
| Filedesc metadata |  emd-17160.cif.gz emd-17160.cif.gz | 7.6 KB | ||
| Others |  emd_17160_additional_1.map.gz emd_17160_additional_1.map.gz emd_17160_half_map_1.map.gz emd_17160_half_map_1.map.gz emd_17160_half_map_2.map.gz emd_17160_half_map_2.map.gz | 37.9 MB 37.7 MB 37.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17160 http://ftp.pdbj.org/pub/emdb/structures/EMD-17160 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17160 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17160 | HTTPS FTP |
-Related structure data
| Related structure data |  8oslMC  8osjC  8oskC  8otsC  8ottC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17160.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17160.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full-map. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.68 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Flexible refinement.
| File | emd_17160_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Flexible refinement. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B.
| File | emd_17160_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A.
| File | emd_17160_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : CLOCK-BMAL1 bound to a nucleosome at SHL -6.2
+Supramolecule #1: CLOCK-BMAL1 bound to a nucleosome at SHL -6.2
+Supramolecule #2: Nucleosome core particle
+Supramolecule #3: Histone octamer
+Supramolecule #4: Nucleosomal DNA
+Supramolecule #5: CLOCK-BMAL1 heterodimer
+Macromolecule #1: Histone H3.1
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A type 1-B/E
+Macromolecule #4: Histone H2B type 1-J
+Macromolecule #7: Circadian locomoter output cycles protein kaput
+Macromolecule #8: Basic helix-loop-helix ARNT-like protein 1
+Macromolecule #5: DNA (147-MER)
+Macromolecule #6: DNA (147-MER)
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Output model |  PDB-8osl: |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)