[English] 日本語
 Yorodumi
Yorodumi- EMDB-16899: 39S human mitochondrial large ribosomal subunit with mtRF1 and P-... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 39S human mitochondrial large ribosomal subunit with mtRF1 and P-site tRNA | ||||||||||||
 Map data Map data | Human 39S mitoribosomal large subunit | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | human mitochondrial ribosome / release factor mtRF1 / non-canonical stop codon / RIBOSOME | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationrRNA import into mitochondrion / mitochondrial transcription / mitochondrial translational termination / Mitochondrial ribosome-associated quality control / mitochondrial translational elongation / Mitochondrial translation elongation / translation release factor activity, codon nonspecific / Mitochondrial translation initiation / Mitochondrial translation termination / translation release factor activity, codon specific ...rRNA import into mitochondrion / mitochondrial transcription / mitochondrial translational termination / Mitochondrial ribosome-associated quality control / mitochondrial translational elongation / Mitochondrial translation elongation / translation release factor activity, codon nonspecific / Mitochondrial translation initiation / Mitochondrial translation termination / translation release factor activity, codon specific / mitochondrial large ribosomal subunit / peptidyl-tRNA hydrolase / translation release factor activity / mitochondrial ribosome / Hydrolases; Acting on ester bonds; Endoribonucleases producing 5'-phosphomonoesters / mitochondrial small ribosomal subunit / peptidyl-tRNA hydrolase activity / mitochondrial translation / anatomical structure morphogenesis / RNA processing / Mitochondrial protein degradation / rescue of stalled cytosolic ribosome / cellular response to leukemia inhibitory factor / fibrillar center / cell junction / double-stranded RNA binding / 5S rRNA binding / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / endonuclease activity / mitochondrial inner membrane / negative regulation of translation / rRNA binding / nuclear body / structural constituent of ribosome / ribosome / translation / mitochondrial matrix / ribonucleoprotein complex / protein domain specific binding / nucleotide binding / mRNA binding / apoptotic process / positive regulation of DNA-templated transcription / nucleolus / mitochondrion / extracellular space / RNA binding / nucleoplasm / nucleus / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
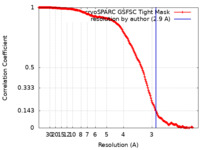
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | ||||||||||||
 Authors Authors | Saurer M / Leibundgut M / Scaiola A / Schoenhut T / Ban N | ||||||||||||
| Funding support |  Switzerland, European Union, 3 items Switzerland, European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Molecular basis of translation termination at noncanonical stop codons in human mitochondria. Authors: Martin Saurer / Marc Leibundgut / Hima Priyanka Nadimpalli / Alain Scaiola / Tanja Schönhut / Richard G Lee / Stefan J Siira / Oliver Rackham / René Dreos / Tea Lenarčič / Eva Kummer / ...Authors: Martin Saurer / Marc Leibundgut / Hima Priyanka Nadimpalli / Alain Scaiola / Tanja Schönhut / Richard G Lee / Stefan J Siira / Oliver Rackham / René Dreos / Tea Lenarčič / Eva Kummer / David Gatfield / Aleksandra Filipovska / Nenad Ban /    Abstract: The genetic code that specifies the identity of amino acids incorporated into proteins during protein synthesis is almost universally conserved. Mitochondrial genomes feature deviations from the ...The genetic code that specifies the identity of amino acids incorporated into proteins during protein synthesis is almost universally conserved. Mitochondrial genomes feature deviations from the standard genetic code, including the reassignment of two arginine codons to stop codons. The protein required for translation termination at these noncanonical stop codons to release the newly synthesized polypeptides is not currently known. In this study, we used gene editing and ribosomal profiling in combination with cryo-electron microscopy to establish that mitochondrial release factor 1 (mtRF1) detects noncanonical stop codons in human mitochondria by a previously unknown mechanism of codon recognition. We discovered that binding of mtRF1 to the decoding center of the ribosome stabilizes a highly unusual conformation in the messenger RNA in which the ribosomal RNA participates in specific recognition of the noncanonical stop codons. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16899.map.gz emd_16899.map.gz | 483.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16899-v30.xml emd-16899-v30.xml emd-16899.xml emd-16899.xml | 84.3 KB 84.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16899_fsc.xml emd_16899_fsc.xml | 16.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_16899.png emd_16899.png | 197.2 KB | ||
| Filedesc metadata |  emd-16899.cif.gz emd-16899.cif.gz | 18.3 KB | ||
| Others |  emd_16899_half_map_1.map.gz emd_16899_half_map_1.map.gz emd_16899_half_map_2.map.gz emd_16899_half_map_2.map.gz | 475.6 MB 475.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16899 http://ftp.pdbj.org/pub/emdb/structures/EMD-16899 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16899 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16899 | HTTPS FTP |
-Related structure data
| Related structure data |  8oitMC  8oinC  8oipC  8oiqC  8oirC  8oisC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16899.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16899.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human 39S mitoribosomal large subunit | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map B
| File | emd_16899_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
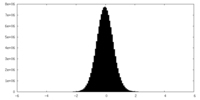
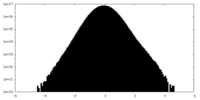
| Density Histograms |
-Half map: half map A
| File | emd_16899_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
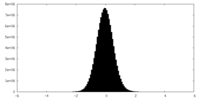
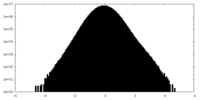
| Density Histograms |
- Sample components
Sample components
+Entire : 39S human mitochondrial ribosome with mtRF1 and P-site tRNA
+Supramolecule #1: 39S human mitochondrial ribosome with mtRF1 and P-site tRNA
+Macromolecule #1: 39S ribosomal protein L12, mitochondrial
+Macromolecule #5: 39S ribosomal protein L22, mitochondrial
+Macromolecule #6: 39S ribosomal protein L23, mitochondrial
+Macromolecule #7: 39S ribosomal protein L24, mitochondrial
+Macromolecule #8: 39S ribosomal protein L27, mitochondrial
+Macromolecule #9: 39S ribosomal protein L28, mitochondrial
+Macromolecule #10: 39S ribosomal protein L47, mitochondrial
+Macromolecule #11: 39S ribosomal protein L30, mitochondrial
+Macromolecule #12: 39S ribosomal protein L32, mitochondrial
+Macromolecule #13: 39S ribosomal protein L33, mitochondrial
+Macromolecule #14: 39S ribosomal protein L34, mitochondrial
+Macromolecule #15: 39S ribosomal protein L35, mitochondrial
+Macromolecule #16: 39S ribosomal protein L2, mitochondrial
+Macromolecule #17: 39S ribosomal protein L3, mitochondrial
+Macromolecule #18: 39S ribosomal protein L4, mitochondrial
+Macromolecule #19: 39S ribosomal protein L9, mitochondrial
+Macromolecule #20: 39S ribosomal protein L10, mitochondrial
+Macromolecule #21: 39S ribosomal protein L11, mitochondrial
+Macromolecule #22: 39S ribosomal protein L13, mitochondrial
+Macromolecule #23: 39S ribosomal protein L14, mitochondrial
+Macromolecule #24: 39S ribosomal protein L15, mitochondrial
+Macromolecule #25: 39S ribosomal protein L16, mitochondrial
+Macromolecule #26: 39S ribosomal protein L17, mitochondrial
+Macromolecule #27: 39S ribosomal protein L18, mitochondrial
+Macromolecule #28: 39S ribosomal protein L19, mitochondrial
+Macromolecule #29: 39S ribosomal protein L20, mitochondrial
+Macromolecule #30: 39S ribosomal protein L21, mitochondrial
+Macromolecule #31: 39S ribosomal protein L52, mitochondrial
+Macromolecule #32: 39S ribosomal protein L53, mitochondrial
+Macromolecule #33: 39S ribosomal protein L54, mitochondrial
+Macromolecule #34: 39S ribosomal protein L55, mitochondrial
+Macromolecule #35: Ribosomal protein 63, mitochondrial
+Macromolecule #36: Peptidyl-tRNA hydrolase ICT1, mitochondrial
+Macromolecule #37: Growth arrest and DNA damage-inducible proteins-interacting protein 1
+Macromolecule #38: 39S ribosomal protein S18a, mitochondrial
+Macromolecule #39: 39S ribosomal protein S30, mitochondrial
+Macromolecule #40: 39S ribosomal protein L1, mitochondrial
+Macromolecule #41: 39S ribosomal protein L36, mitochondrial
+Macromolecule #42: 39S ribosomal protein L37, mitochondrial
+Macromolecule #43: 39S ribosomal protein L38, mitochondrial
+Macromolecule #44: 39S ribosomal protein L39, mitochondrial
+Macromolecule #45: 39S ribosomal protein L40, mitochondrial
+Macromolecule #46: 39S ribosomal protein L41, mitochondrial
+Macromolecule #47: 39S ribosomal protein L42, mitochondrial
+Macromolecule #48: Large ribosomal subunit protein mL43
+Macromolecule #49: 39S ribosomal protein L44, mitochondrial
+Macromolecule #50: 39S ribosomal protein L45, mitochondrial
+Macromolecule #51: 39S ribosomal protein L46, mitochondrial
+Macromolecule #52: 39S ribosomal protein L48, mitochondrial
+Macromolecule #53: 39S ribosomal protein L49, mitochondrial
+Macromolecule #54: 39S ribosomal protein L50, mitochondrial
+Macromolecule #55: 39S ribosomal protein L51, mitochondrial
+Macromolecule #57: Peptide chain release factor 1, mitochondrial
+Macromolecule #2: E-site tRNA
+Macromolecule #3: 16S rRNA
+Macromolecule #4: CP Val-tRNA(Val)
+Macromolecule #56: P-site Met-tRNA(Met)
+Macromolecule #58: POTASSIUM ION
+Macromolecule #59: MAGNESIUM ION
+Macromolecule #60: VALINE
+Macromolecule #61: ZINC ION
+Macromolecule #62: FE-S-O HYBRID CLUSTER
+Macromolecule #63: METHIONINE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||
| Details | Affinity-purified 55S mitoribosome. Refinement focused on 39S large subunit. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 2 / Number real images: 43882 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT | |||||||||
| Output model |  PDB-8oit: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)