[English] 日本語
 Yorodumi
Yorodumi- EMDB-16382: Transmembrane domain of resting state homomeric GluA2 F231A mutan... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Transmembrane domain of resting state homomeric GluA2 F231A mutant AMPA receptor in complex with TARP gamma 2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AMPAR / ion channels / neurotransmission / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationPresynaptic depolarization and calcium channel opening / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / channel regulator activity / LGI-ADAM interactions / Trafficking of AMPA receptors / regulation of AMPA receptor activity / membrane hyperpolarization ...Presynaptic depolarization and calcium channel opening / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / channel regulator activity / LGI-ADAM interactions / Trafficking of AMPA receptors / regulation of AMPA receptor activity / membrane hyperpolarization / nervous system process / protein targeting to membrane / voltage-gated calcium channel complex / spine synapse / dendritic spine cytoplasm / dendritic spine neck / cellular response to amine stimulus / dendritic spine head / neurotransmitter receptor localization to postsynaptic specialization membrane / Activation of AMPA receptors / ligand-gated monoatomic cation channel activity / perisynaptic space / neuromuscular junction development / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / response to lithium ion / transmission of nerve impulse / AMPA glutamate receptor clustering / cellular response to glycine / kainate selective glutamate receptor activity / immunoglobulin binding / asymmetric synapse / AMPA glutamate receptor complex / regulation of receptor recycling / extracellularly glutamate-gated ion channel activity / ionotropic glutamate receptor complex / membrane depolarization / conditioned place preference / Unblocking of NMDA receptors, glutamate binding and activation / glutamate receptor binding / positive regulation of synaptic transmission / regulation of synaptic transmission, glutamatergic / regulation of postsynaptic membrane neurotransmitter receptor levels / positive regulation of synaptic transmission, glutamatergic / response to fungicide / voltage-gated calcium channel activity / cytoskeletal protein binding / extracellular ligand-gated monoatomic ion channel activity / glutamate-gated receptor activity / cellular response to brain-derived neurotrophic factor stimulus / regulation of long-term synaptic depression / somatodendritic compartment / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / dendrite membrane / ionotropic glutamate receptor binding / excitatory synapse / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / synaptic membrane / positive regulation of excitatory postsynaptic potential / dendritic shaft / hippocampal mossy fiber to CA3 synapse / SNARE binding / PDZ domain binding / calcium channel regulator activity / regulation of membrane potential / protein tetramerization / synaptic transmission, glutamatergic / establishment of protein localization / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / response to calcium ion / cerebral cortex development / receptor internalization / postsynaptic density membrane / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / terminal bouton / synaptic vesicle / long-term synaptic potentiation / synaptic vesicle membrane / signaling receptor activity / amyloid-beta binding / presynapse / growth cone / presynaptic membrane / scaffold protein binding / dendritic spine / chemical synaptic transmission / perikaryon / postsynaptic membrane / neuron projection / postsynaptic density / external side of plasma membrane / axon / neuronal cell body / dendrite / synapse / protein kinase binding Similarity search - Function | |||||||||
| Biological species |   | |||||||||
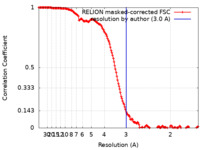
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Zhang D / Ivica J / Krieger JM / Ho H / Yamashita K / Cais O / Greger I | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural mobility tunes signalling of the GluA1 AMPA glutamate receptor. Authors: Danyang Zhang / Josip Ivica / James M Krieger / Hinze Ho / Keitaro Yamashita / Imogen Stockwell / Rozbeh Baradaran / Ondrej Cais / Ingo H Greger /   Abstract: AMPA glutamate receptors (AMPARs), the primary mediators of excitatory neurotransmission in the brain, are either GluA2 subunit-containing and thus Ca-impermeable, or GluA2-lacking and Ca-permeable. ...AMPA glutamate receptors (AMPARs), the primary mediators of excitatory neurotransmission in the brain, are either GluA2 subunit-containing and thus Ca-impermeable, or GluA2-lacking and Ca-permeable. Despite their prominent expression throughout interneurons and glia, their role in long-term potentiation and their involvement in a range of neuropathologies, structural information for GluA2-lacking receptors is currently absent. Here we determine and characterize cryo-electron microscopy structures of the GluA1 homotetramer, fully occupied with TARPγ3 auxiliary subunits (GluA1/γ3). The gating core of both resting and open-state GluA1/γ3 closely resembles GluA2-containing receptors. However, the sequence-diverse N-terminal domains (NTDs) give rise to a highly mobile assembly, enabling domain swapping and subunit re-alignments in the ligand-binding domain tier that are pronounced in desensitized states. These transitions underlie the unique kinetic properties of GluA1. A GluA2 mutant (F231A) increasing NTD dynamics phenocopies this behaviour, and exhibits reduced synaptic responses, reflecting the anchoring function of the AMPAR NTD at the synapse. Together, this work underscores how the subunit-diverse NTDs determine subunit arrangement, gating properties and ultimately synaptic signalling efficiency among AMPAR subtypes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16382.map.gz emd_16382.map.gz | 12.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16382-v30.xml emd-16382-v30.xml emd-16382.xml emd-16382.xml | 17.7 KB 17.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16382_fsc.xml emd_16382_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_16382.png emd_16382.png | 49.2 KB | ||
| Masks |  emd_16382_msk_1.map emd_16382_msk_1.map | 107.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16382.cif.gz emd-16382.cif.gz | 6.6 KB | ||
| Others |  emd_16382_half_map_1.map.gz emd_16382_half_map_1.map.gz emd_16382_half_map_2.map.gz emd_16382_half_map_2.map.gz | 99 MB 99 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16382 http://ftp.pdbj.org/pub/emdb/structures/EMD-16382 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16382 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16382 | HTTPS FTP |
-Related structure data
| Related structure data |  8c1sMC  8c1pC  8c1qC  8c1rC  8c2hC  8c2iC  8p3qC  8p3sC  8p3tC  8p3uC  8p3vC  8p3wC  8p3xC  8p3yC  8p3zC  8pivC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16382.map.gz / Format: CCP4 / Size: 107.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16382.map.gz / Format: CCP4 / Size: 107.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||
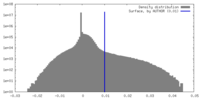
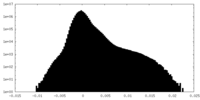
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16382_msk_1.map emd_16382_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
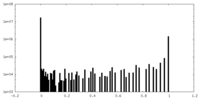
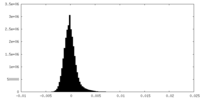
| Density Histograms |
-Half map: #2
| File | emd_16382_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
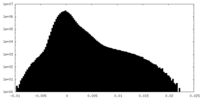
| Density Histograms |
-Half map: #1
| File | emd_16382_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
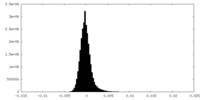
| Density Histograms |
- Sample components
Sample components
-Entire : homomeric GluA2 F231A mutant AMPA receptor in complex with TARP g...
| Entire | Name: homomeric GluA2 F231A mutant AMPA receptor in complex with TARP gamma 2 |
|---|---|
| Components |
|
-Supramolecule #1: homomeric GluA2 F231A mutant AMPA receptor in complex with TARP g...
| Supramolecule | Name: homomeric GluA2 F231A mutant AMPA receptor in complex with TARP gamma 2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Glutamate receptor 2
| Macromolecule | Name: Glutamate receptor 2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 99.981031 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MQKIMHISVL LSPVLWGLIF GDYKDDDDKV SSNSIQIGGL FPRGADQEYS AFRVGMVQFS TSEFRLTPHI DNLEVANSFA VTNAFCSQF SRGVYAIFGF YDKKSVNTIT SFCGTLHVSF ITPSFPTDGT HPFVIQMRPD LKGALLSLIE YYQWDKFAYL Y DSDRGLST ...String: MQKIMHISVL LSPVLWGLIF GDYKDDDDKV SSNSIQIGGL FPRGADQEYS AFRVGMVQFS TSEFRLTPHI DNLEVANSFA VTNAFCSQF SRGVYAIFGF YDKKSVNTIT SFCGTLHVSF ITPSFPTDGT HPFVIQMRPD LKGALLSLIE YYQWDKFAYL Y DSDRGLST LQAVLDSAAE KKWQVTAINV GNINNDKKDE TYRSLFQDLE LKKERRVILD CERDKVNDIV DQVITIGKHV KG YHYIIAN LGFTDGDLLK IQFGGANVSG FQIVDYDDSL VSKFIERWST LEEKEYPGAH TATIKYTSAL TYDAVQVMTE AFR NLRKQR IEISRRGNAG DCLANPAVPW GQGVEIERAL KQVQVEGLSG NIKFDQNGKR INYTINIMEL KTNGPRKIGY WSEV DKMVV TLTELPSGND TSGLENKTVV VTTILESPYV MMKKNHEMLE GNERYEGYCV DLAAEIAKHC GFKYKLTIVG DGKYG ARDA DTKIWNGMVG ELVYGKADIA IAPLTITLVR EEVIDFSKPF MSLGISIMIK KPQKSKPGVF SFLDPLAYEI WMCIVF AYI GVSVVLFLVS RFSPYEWHTE EFEDGRETQS SESTNEFGIF NSLWFSLGAF MRQGCDISPR SLSGRIVGGV WWFFTLI II SSYTANLAAF LTVERMVSPI ESAEDLSKQT EIAYGTLDSG STKEFFRRSK IAVFDKMWTY MRSAEPSVFV RTTAEGVA R VRKSKGKYAY LLESTMNEYI EQRKPCDTMK VGGNLDSKGY GIATPKGSSL RTPVNLAVLK LSEQGVLDKL KNKWWYDKG ECGAKDSGSK EKTSALSLSN VAGVFYILVG GLGLAMLVAL IEFCYKSRAE AKRMKVAKNP QNINPSSSQN SQNFATYKEG YNVYGIESV KI UniProtKB: Glutamate receptor 2 |
-Macromolecule #2: Voltage-dependent calcium channel gamma-2 subunit
| Macromolecule | Name: Voltage-dependent calcium channel gamma-2 subunit / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 35.807555 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GLFDRGVQML LTTVGAFAAF SLMTIAVGTD YWLYSRGVCK TKSVSENETS KKNEEVMTHS GLWRTCCLEG NFKGLCKQID HFPEDADYE ADTAEYFLRA VRASSIFPIL SVILLFMGGL CIAASEFYKT RHNIILSAGI FFVSAGLSNI IGIIVYISAN A GDPSKSDS ...String: GLFDRGVQML LTTVGAFAAF SLMTIAVGTD YWLYSRGVCK TKSVSENETS KKNEEVMTHS GLWRTCCLEG NFKGLCKQID HFPEDADYE ADTAEYFLRA VRASSIFPIL SVILLFMGGL CIAASEFYKT RHNIILSAGI FFVSAGLSNI IGIIVYISAN A GDPSKSDS KKNSYSYGWS FYFGALSFII AEMVGVLAVH MFIDRHKQLR ATARATDYLQ ASAITRIPSY RYRYQRRSRS SS RSTEPSH SRDASPVGVK GFNTLPSTEI SMYTLSRDPL KAATTPTATY NSDRDNSFLQ VHNCIQKDSK DSLHANTANR RTT PV UniProtKB: Voltage-dependent calcium channel gamma-2 subunit |
-Macromolecule #3: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 3 / Number of copies: 6 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Macromolecule #4: (2R)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate
| Macromolecule | Name: (2R)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate / type: ligand / ID: 4 / Number of copies: 4 / Formula: OLC |
|---|---|
| Molecular weight | Theoretical: 356.54 Da |
| Chemical component information |  ChemComp-OLC: |
-Macromolecule #5: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 5 / Number of copies: 4 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 8 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.4000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Servalcat |
|---|---|
| Refinement | Space: RECIPROCAL |
| Output model |  PDB-8c1s: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)