[English] 日本語
 Yorodumi
Yorodumi- EMDB-15309: Pol alpha - replisome complex containing Ctf4. Engaged on a DNA f... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pol alpha - replisome complex containing Ctf4. Engaged on a DNA fork containing a 60 nucleotide lagging strand. | |||||||||
 Map data Map data | Pol alpha - replisome complex containing Ctf4. Unsharpened. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Replication / helicase / polymerase / pol alpha / priming | |||||||||
| Function / homology |  Function and homology information Function and homology informationInhibition of replication initiation of damaged DNA by RB1/E2F1 / establishment of sister chromatid cohesion / H3-H4 histone complex chaperone activity / Unwinding of DNA / maintenance of DNA repeat elements / regulation of nuclear cell cycle DNA replication / DNA replication initiation / replication fork arrest / Cul8-RING ubiquitin ligase complex / meiotic chromosome segregation ...Inhibition of replication initiation of damaged DNA by RB1/E2F1 / establishment of sister chromatid cohesion / H3-H4 histone complex chaperone activity / Unwinding of DNA / maintenance of DNA repeat elements / regulation of nuclear cell cycle DNA replication / DNA replication initiation / replication fork arrest / Cul8-RING ubiquitin ligase complex / meiotic chromosome segregation / RNA-templated DNA biosynthetic process / Processive synthesis on the lagging strand / Removal of the Flap Intermediate / MCM core complex / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / DNA strand elongation involved in mitotic DNA replication / GINS complex / MCM complex binding / mitotic DNA replication preinitiation complex assembly / nuclear DNA replication / Polymerase switching / premeiotic DNA replication / replication fork protection complex / pre-replicative complex assembly involved in nuclear cell cycle DNA replication / alpha DNA polymerase:primase complex / anaphase-promoting complex binding / Activation of the pre-replicative complex / mitotic DNA replication / CMG complex / DNA replication checkpoint signaling / establishment of mitotic sister chromatid cohesion / nuclear pre-replicative complex / DNA replication preinitiation complex / Activation of ATR in response to replication stress / MCM complex / mitotic DNA replication checkpoint signaling / lagging strand elongation / double-strand break repair via break-induced replication / mitotic DNA replication initiation / DNA replication, synthesis of primer / telomere capping / cellular response to osmotic stress / single-stranded DNA helicase activity / mitotic intra-S DNA damage checkpoint signaling / silent mating-type cassette heterochromatin formation / regulation of DNA-templated DNA replication initiation / mitotic sister chromatid cohesion / DNA biosynthetic process / DNA strand elongation involved in DNA replication / nuclear chromosome / DNA synthesis involved in DNA repair / leading strand elongation / mitotic G2 DNA damage checkpoint signaling / nuclear replication fork / replication fork processing / DNA replication origin binding / DNA replication initiation / Ub-specific processing proteases / subtelomeric heterochromatin formation / nuclear periphery / DNA helicase activity / telomere maintenance / replication fork / meiotic cell cycle / transcription elongation by RNA polymerase II / helicase activity / DNA-templated DNA replication / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / DNA-directed RNA polymerase activity / nuclear envelope / heterochromatin formation / mitotic cell cycle / double-strand break repair / nucleosome assembly / single-stranded DNA binding / 4 iron, 4 sulfur cluster binding / DNA-directed DNA polymerase / DNA helicase / DNA-directed DNA polymerase activity / DNA replication / chromosome, telomeric region / protein stabilization / nucleotide binding / DNA repair / DNA damage response / chromatin binding / ATP hydrolysis activity / mitochondrion / DNA binding / zinc ion binding / nucleoplasm / ATP binding / metal ion binding / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
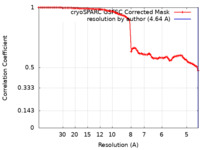
| Method | single particle reconstruction / cryo EM / Resolution: 4.64 Å | |||||||||
 Authors Authors | Jones ML / Yeeles JTP | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: How Pol α-primase is targeted to replisomes to prime eukaryotic DNA replication. Authors: Morgan L Jones / Valentina Aria / Yasemin Baris / Joseph T P Yeeles /  Abstract: During eukaryotic DNA replication, Pol α-primase generates primers at replication origins to start leading-strand synthesis and every few hundred nucleotides during discontinuous lagging-strand ...During eukaryotic DNA replication, Pol α-primase generates primers at replication origins to start leading-strand synthesis and every few hundred nucleotides during discontinuous lagging-strand replication. How Pol α-primase is targeted to replication forks to prime DNA synthesis is not fully understood. Here, by determining cryoelectron microscopy (cryo-EM) structures of budding yeast and human replisomes containing Pol α-primase, we reveal a conserved mechanism for the coordination of priming by the replisome. Pol α-primase binds directly to the leading edge of the CMG (CDC45-MCM-GINS) replicative helicase via a complex interaction network. The non-catalytic PRIM2/Pri2 subunit forms two interfaces with CMG that are critical for in vitro DNA replication and yeast cell growth. These interactions position the primase catalytic subunit PRIM1/Pri1 directly above the exit channel for lagging-strand template single-stranded DNA (ssDNA), revealing why priming occurs efficiently only on the lagging-strand template and elucidating a mechanism for Pol α-primase to overcome competition from RPA to initiate primer synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15309.map.gz emd_15309.map.gz | 20.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15309-v30.xml emd-15309-v30.xml emd-15309.xml emd-15309.xml | 13.9 KB 13.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15309_fsc.xml emd_15309_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_15309.png emd_15309.png | 100.9 KB | ||
| Others |  emd_15309_additional_1.map.gz emd_15309_additional_1.map.gz emd_15309_half_map_1.map.gz emd_15309_half_map_1.map.gz emd_15309_half_map_2.map.gz emd_15309_half_map_2.map.gz | 2 MB 37.7 MB 37.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15309 http://ftp.pdbj.org/pub/emdb/structures/EMD-15309 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15309 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15309 | HTTPS FTP |
-Related structure data
| Related structure data |  8b9aMC  8b9bMC  8b9cC  8b9dC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15309.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15309.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Pol alpha - replisome complex containing Ctf4. Unsharpened. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.26727 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Locally filtered map
| File | emd_15309_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally filtered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map
| File | emd_15309_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map
| File | emd_15309_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Replisome - pol alpha complex
| Entire | Name: Replisome - pol alpha complex |
|---|---|
| Components |
|
-Supramolecule #1: Replisome - pol alpha complex
| Supramolecule | Name: Replisome - pol alpha complex / type: complex / ID: 1 / Parent: 0 Details: S. cerevisiae pol alpha bound to the core replisome engaged with a fork DNA substrate containing a 60 nucleotide lagging strand. |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.184 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)