[English] 日本語
 Yorodumi
Yorodumi- EMDB-12721: Yeast TFIIH in the contracted state within the pre-initiation complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12721 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Yeast TFIIH in the contracted state within the pre-initiation complex | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Pre-initiation complex / TRANSCRIPTION | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of mitotic recombination / transcription open complex formation at RNA polymerase II promoter / phosphatidylinositol-5-phosphate binding / RNA polymerase II promoter clearance / positive regulation of mitotic recombination / transcription factor TFIIE complex / nucleotide-excision repair factor 3 complex / nucleotide-excision repair, preincision complex assembly / DNA translocase activity / transcription factor TFIIK complex ...regulation of mitotic recombination / transcription open complex formation at RNA polymerase II promoter / phosphatidylinositol-5-phosphate binding / RNA polymerase II promoter clearance / positive regulation of mitotic recombination / transcription factor TFIIE complex / nucleotide-excision repair factor 3 complex / nucleotide-excision repair, preincision complex assembly / DNA translocase activity / transcription factor TFIIK complex / transcriptional start site selection at RNA polymerase II promoter / RPB4-RPB7 complex / phosphatidylinositol-3-phosphate binding / DNA 5'-3' helicase / transcription factor TFIIH core complex / transcription factor TFIIH holo complex / cyclin-dependent protein serine/threonine kinase activator activity / nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / RNA Polymerase I Transcription Initiation / transcription preinitiation complex / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Pol II CTD phosphorylation and interaction with CE / Formation of the Early Elongation Complex / mRNA Capping / RNA polymerase II transcribes snRNA genes / TP53 Regulates Transcription of DNA Repair Genes / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / poly(A)+ mRNA export from nucleus / termination of RNA polymerase III transcription / RNA Polymerase II Pre-transcription Events / RNA-templated transcription / Formation of TC-NER Pre-Incision Complex / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / 3'-5' DNA helicase activity / RNA Polymerase I Promoter Escape / transcription initiation at RNA polymerase III promoter / DNA 3'-5' helicase / termination of RNA polymerase I transcription / Gap-filling DNA repair synthesis and ligation in TC-NER / nucleolar large rRNA transcription by RNA polymerase I / transcription initiation at RNA polymerase I promoter / Estrogen-dependent gene expression / RNA polymerase II complex binding / ATPase activator activity / transcription by RNA polymerase III / positive regulation of translational initiation / Dual incision in TC-NER / nuclear-transcribed mRNA catabolic process / ATP-dependent activity, acting on DNA / RNA polymerase I complex / RNA polymerase III complex / transcription elongation by RNA polymerase I / translesion synthesis / RNA polymerase II, core complex / tRNA transcription by RNA polymerase III / transcription by RNA polymerase I / translation initiation factor binding / DNA helicase activity / nucleotide-excision repair / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / P-body / DNA-templated transcription initiation / mRNA transcription by RNA polymerase II / ribonucleoside binding / mRNA processing / DNA-directed RNA polymerase / cytoplasmic stress granule / DNA-directed RNA polymerase activity / ubiquitin protein ligase activity / single-stranded DNA binding / ribosome biogenesis / 4 iron, 4 sulfur cluster binding / double-stranded DNA binding / 5'-3' DNA helicase activity / transcription by RNA polymerase II / damaged DNA binding / single-stranded RNA binding / nucleotide binding / DNA repair / mRNA binding / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / ATP hydrolysis activity / mitochondrion / DNA binding / zinc ion binding / nucleoplasm / ATP binding / metal ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||||||||
 Authors Authors | Schilbach S / Aibara S | ||||||||||||||||||
| Funding support |  Germany, 5 items Germany, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: Structure of RNA polymerase II pre-initiation complex at 2.9 Å defines initial DNA opening. Authors: Sandra Schilbach / Shintaro Aibara / Christian Dienemann / Frauke Grabbe / Patrick Cramer /  Abstract: Transcription initiation requires assembly of the RNA polymerase II (Pol II) pre-initiation complex (PIC) and opening of promoter DNA. Here, we present the long-sought high-resolution structure of ...Transcription initiation requires assembly of the RNA polymerase II (Pol II) pre-initiation complex (PIC) and opening of promoter DNA. Here, we present the long-sought high-resolution structure of the yeast PIC and define the mechanism of initial DNA opening. We trap the PIC in an intermediate state that contains half a turn of open DNA located 30-35 base pairs downstream of the TATA box. The initially opened DNA region is flanked and stabilized by the polymerase "clamp head loop" and the TFIIF "charged region" that both contribute to promoter-initiated transcription. TFIIE facilitates initiation by buttressing the clamp head loop and by regulating the TFIIH translocase. The initial DNA bubble is then extended in the upstream direction, leading to the open promoter complex and enabling start-site scanning and RNA synthesis. This unique mechanism of DNA opening may permit more intricate regulation than in the Pol I and Pol III systems. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
-Related structure data
| Related structure data |  7o4kMC  7o4iC  7o4jC  7o4lC  7o72C  7o73C  7o75C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12721.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12721.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
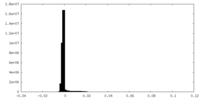
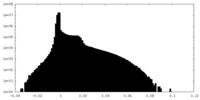
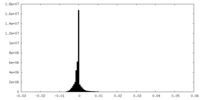
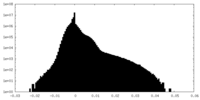
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
+Mask #1
+Additional map: Locally filtered map (non-composite) of a refinement of...
+Additional map: Half-map 2 of focused refinement encompassing the Tfb2...
+Additional map: Half-map 2 of focused refinement encompassing the Ssl2/Tfb2/Tfb5...
+Additional map: Half-map 1 of focused refinement encompassing the Tfb2...
+Additional map: Half-map 1 of focused refinement encompassing the Ssl2/Tfb2/Tfb5...
+Additional map: Half-map 1 of focused refinement encompassing the Tfb3-RING-finger/TFIIE/Pol...
+Additional map: Half-map 2 of focused refinement encompassing the Tfb3-RING-finger/TFIIE/Pol...
+Additional map: Half-map 1 of focused refinement encompassing the Pol...
+Additional map: Half-map 1 of focused refinement encompassing the Pol...
+Additional map: Half-map 2 of focused refinement encompassing the Pol...
+Additional map: Half-map 2 of focused refinement encompassing the Pol...
+Additional map: Half-map 1 of focused refinement encompassing the Rad3/Ssl1...
+Additional map: Half-map 2 of focused refinement encompassing the Rad3/Ssl1...
+Additional map: Half-map 1 of focused refinement encompassing the Ssl1/Tfb4-eZnF/Tfb1-3-helix-bundle...
+Additional map: Half-map 2 of focused refinement encompassing the Ssl1/Tfb4-eZnF/Tfb1-3-helix-bundle...
+Half map: #2
+Half map: #1
- Sample components
Sample components
+Entire : Yeast TFIIH in the contracted state within the pre-initiation complex
+Supramolecule #1: Yeast TFIIH in the contracted state within the pre-initiation complex
+Macromolecule #1: General transcription and DNA repair factor IIH helicase subunit XPD
+Macromolecule #2: General transcription and DNA repair factor IIH subunit TFB1
+Macromolecule #3: General transcription and DNA repair factor IIH subunit TFB2
+Macromolecule #4: RNA polymerase II transcription factor B subunit 3
+Macromolecule #5: General transcription and DNA repair factor IIH subunit TFB4
+Macromolecule #6: General transcription and DNA repair factor IIH subunit TFB5
+Macromolecule #7: General transcription and DNA repair factor IIH subunit SSL1
+Macromolecule #8: General transcription and DNA repair factor IIH helicase subunit XPB
+Macromolecule #9: DNA-directed RNA polymerase II subunit RPB1
+Macromolecule #10: DNA-directed RNA polymerase II subunit RPB2
+Macromolecule #11: DNA-directed RNA polymerase II subunit RPB4
+Macromolecule #12: DNA-directed RNA polymerases I, II, and III subunit RPABC2
+Macromolecule #13: DNA-directed RNA polymerase II subunit RPB7
+Macromolecule #16: Transcription initiation factor IIE subunit alpha
+Macromolecule #17: Transcription initiation factor IIE subunit beta
+Macromolecule #14: Non-template DNA
+Macromolecule #15: Template DNA
+Macromolecule #18: IRON/SULFUR CLUSTER
+Macromolecule #19: ZINC ION
+Macromolecule #20: BERYLLIUM TRIFLUORIDE ION
+Macromolecule #21: MAGNESIUM ION
+Macromolecule #22: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Grid | Model: Quantifoil R3.5/1 / Material: COPPER / Support film - Material: CARBON / Support film - topology: CONTINUOUS |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 29670 / Average exposure time: 9.0 sec. / Average electron dose: 43.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 130000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)






































































































































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)