[English] 日本語
 Yorodumi
Yorodumi- EMDB-12210: Formate dehydrogenase - heterodisulfide reductase - formylmethano... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12210 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Formate dehydrogenase - heterodisulfide reductase - formylmethanofuran dehydrogenase complex from Methanospirillum hungatei (heterodislfide reductase core and mobile arm in conformational state 1, composite structure) | |||||||||||||||
 Map data Map data | Composite map of the heterodislfide reductase core and mobile arm in state 1 of the formate dehydrogenase - heterodisulfide reductase - formylmethanofuran dehydrogenase complex from M. hungatei | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | methanogenesis / flavin-based electron bifurcation / CO2-fixation / formate dehydrogenase / OXIDOREDUCTASE | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationOxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors / CoB--CoM heterodisulfide reductase activity / oxidoreductase activity, acting on CH or CH2 groups, with an iron-sulfur protein as acceptor / formate metabolic process / methanogenesis / formate dehydrogenase (NAD+) activity / Oxidoreductases; Acting on a sulfur group of donors / molybdopterin cofactor binding / NADH dehydrogenase activity / iron-sulfur cluster binding ...Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors / CoB--CoM heterodisulfide reductase activity / oxidoreductase activity, acting on CH or CH2 groups, with an iron-sulfur protein as acceptor / formate metabolic process / methanogenesis / formate dehydrogenase (NAD+) activity / Oxidoreductases; Acting on a sulfur group of donors / molybdopterin cofactor binding / NADH dehydrogenase activity / iron-sulfur cluster binding / respiratory electron transport chain / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / metal ion binding / membrane / plasma membrane Similarity search - Function | |||||||||||||||
| Biological species |  Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||||||||
 Authors Authors | Pfeil-Gardiner O / Watanabe T | |||||||||||||||
| Funding support |  Germany, Germany,  Japan, 4 items Japan, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Three-megadalton complex of methanogenic electron-bifurcating and CO-fixing enzymes. Authors: Tomohiro Watanabe / Olivia Pfeil-Gardiner / Jörg Kahnt / Jürgen Koch / Seigo Shima / Bonnie J Murphy /  Abstract: The first reaction of the methanogenic pathway from carbon dioxide (CO) is the reduction and condensation of CO to formyl-methanofuran, catalyzed by formyl-methanofuran dehydrogenase (Fmd). Strongly ...The first reaction of the methanogenic pathway from carbon dioxide (CO) is the reduction and condensation of CO to formyl-methanofuran, catalyzed by formyl-methanofuran dehydrogenase (Fmd). Strongly reducing electrons for this reaction are generated by heterodisulfide reductase (Hdr) in complex with hydrogenase or formate dehydrogenase (Fdh) using a flavin-based electron-bifurcation mechanism. Here, we report enzymological and structural characterizations of Fdh-Hdr-Fmd complexes from . The complexes catalyze this reaction using electrons from formate and the reduced form of the electron carrier F. Conformational changes in HdrA mediate electron bifurcation, and polyferredoxin FmdF directly transfers electrons to the CO reduction site, as evidenced by methanofuran-dependent flavin-based electron bifurcation even without free ferredoxin, a diffusible electron carrier between Hdr and Fmd. Conservation of Hdr and Fmd structures suggests that this complex is common among hydrogenotrophic methanogens. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12210.map.gz emd_12210.map.gz | 48.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12210-v30.xml emd-12210-v30.xml emd-12210.xml emd-12210.xml | 25.6 KB 25.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_12210.png emd_12210.png | 195.4 KB | ||
| Filedesc metadata |  emd-12210.cif.gz emd-12210.cif.gz | 8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12210 http://ftp.pdbj.org/pub/emdb/structures/EMD-12210 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12210 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12210 | HTTPS FTP |
-Related structure data
| Related structure data |  7bkdMC  7bkbC  7bkcC  7bkeC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12210.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12210.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of the heterodislfide reductase core and mobile arm in state 1 of the formate dehydrogenase - heterodisulfide reductase - formylmethanofuran dehydrogenase complex from M. hungatei | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.837 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
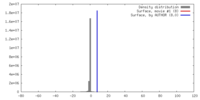
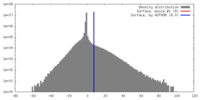
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Dimeric formate dehydrogenase - heterodisulfide reductase - formy...
+Supramolecule #1: Dimeric formate dehydrogenase - heterodisulfide reductase - formy...
+Macromolecule #1: CoB--CoM heterodisulfide reductase iron-sulfur subunit A
+Macromolecule #2: CoB--CoM heterodisulfide reductase subunit C
+Macromolecule #3: CoB--CoM heterodisulfide reductase subunit B
+Macromolecule #4: F420-non-reducing hydrogenase subunit D
+Macromolecule #5: Formate dehydrogenase, beta subunit (F420)
+Macromolecule #6: Formate dehydrogenase
+Macromolecule #7: IRON/SULFUR CLUSTER
+Macromolecule #8: FLAVIN-ADENINE DINUCLEOTIDE
+Macromolecule #9: Non-cubane [4Fe-4S]-cluster
+Macromolecule #10: FE2/S2 (INORGANIC) CLUSTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.6 / Component - Concentration: 25.0 mM / Component - Formula: Tris-HCl / Component - Name: Tris-HCl |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 70 % / Chamber temperature: 293 K / Instrument: HOMEMADE PLUNGER |
| Details | Preparation in an anaerobic tent (O2 < 20 ppm at all times, nearly always < 2ppm) |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 8745 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|---|
| Output model |  PDB-7bkd: |
 Movie
Movie Controller
Controller



































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)