+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10123 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of ZEBOV GP in complex with 3T0265 antibody | |||||||||
 Map data Map data | Local filtered, Z flipped | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ebola / glycoprotein / antibodies / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated killing of host cell / host cell endoplasmic reticulum / viral budding from plasma membrane / clathrin-dependent endocytosis of virus by host cell / symbiont-mediated-mediated suppression of host tetherin activity / entry receptor-mediated virion attachment to host cell / host cell cytoplasm / symbiont-mediated suppression of host innate immune response / membrane raft / fusion of virus membrane with host endosome membrane ...symbiont-mediated killing of host cell / host cell endoplasmic reticulum / viral budding from plasma membrane / clathrin-dependent endocytosis of virus by host cell / symbiont-mediated-mediated suppression of host tetherin activity / entry receptor-mediated virion attachment to host cell / host cell cytoplasm / symbiont-mediated suppression of host innate immune response / membrane raft / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / lipid binding / host cell plasma membrane / virion membrane / extracellular region / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
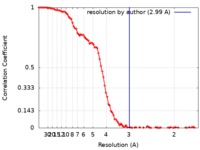
| Method | single particle reconstruction / cryo EM / Resolution: 2.99 Å | |||||||||
 Authors Authors | Diskin R / Cohen-Dvashi H | |||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2020 Journal: Cell Host Microbe / Year: 2020Title: Structural Basis for a Convergent Immune Response against Ebola Virus. Authors: Hadas Cohen-Dvashi / Matthias Zehner / Stefanie Ehrhardt / Michael Katz / Nadav Elad / Florian Klein / Ron Diskin /   Abstract: Ebola virus disease is a severe health problem in Africa. Vaccines that display the Zaire ebolavirus glycoprotein spike complex are a prime component for the effort to combat it. The V3-15/V1-40- ...Ebola virus disease is a severe health problem in Africa. Vaccines that display the Zaire ebolavirus glycoprotein spike complex are a prime component for the effort to combat it. The V3-15/V1-40-based class of antibodies was recently discovered to be a common response in individuals who received the Ebola virus vaccines. These antibodies display attractive properties, and thus likely contribute to the efficacy of the vaccines. Here, we use cryo-EM to elucidate how three V3-15/V1-40 antibodies from different individuals target the virus and found a convergent mechanism against a partially conserved site on the spike complex. Our study rationalizes the selection of the V3-15/V1-40 germline genes for specifically targeting this site and highlights Ebolavirus species-specific sequence divergences that may restrict breadth of V3-15/V1-40-based humoral response. The results from this study could help develop improved immunization schemes and further enable the design of immunogens that would be efficacious against a broader set of Ebolavirus species. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10123.map.gz emd_10123.map.gz | 5.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10123-v30.xml emd-10123-v30.xml emd-10123.xml emd-10123.xml | 19.3 KB 19.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10123_fsc.xml emd_10123_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_10123.png emd_10123.png | 135.8 KB | ||
| Filedesc metadata |  emd-10123.cif.gz emd-10123.cif.gz | 6.1 KB | ||
| Others |  emd_10123_half_map_1.map.gz emd_10123_half_map_1.map.gz emd_10123_half_map_2.map.gz emd_10123_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10123 http://ftp.pdbj.org/pub/emdb/structures/EMD-10123 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10123 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10123 | HTTPS FTP |
-Related structure data
| Related structure data |  6s8iMC  6s8dC  6s8jC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10123.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10123.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local filtered, Z flipped | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.849 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Half map A
| File | emd_10123_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_10123_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : EBOV-GP in complex with 3T0265 antibody
| Entire | Name: EBOV-GP in complex with 3T0265 antibody |
|---|---|
| Components |
|
-Supramolecule #1: EBOV-GP in complex with 3T0265 antibody
| Supramolecule | Name: EBOV-GP in complex with 3T0265 antibody / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: EBOV-GP
| Supramolecule | Name: EBOV-GP / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #3-#4 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: 3T0265 antibody
| Supramolecule | Name: 3T0265 antibody / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #1-#2 / Details: A fab portion generated by cleavage |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Light Chain
| Macromolecule | Name: Light Chain / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.080494 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVLTQPPSV SGAPGQRVAI SCTGSYSNIG AGYDVHWYQQ FPGAAPKLLI YAYTNRPAGV PDRFSGSKSG SSASLVISGL QADDEADYY CQSYDARLRD GEVFGGGTKL TVLGQPKAAP SVTLFPPSSE ELQANKATLV CLISDFYPGA VTVAWKADSS P VKAGVETT ...String: QSVLTQPPSV SGAPGQRVAI SCTGSYSNIG AGYDVHWYQQ FPGAAPKLLI YAYTNRPAGV PDRFSGSKSG SSASLVISGL QADDEADYY CQSYDARLRD GEVFGGGTKL TVLGQPKAAP SVTLFPPSSE ELQANKATLV CLISDFYPGA VTVAWKADSS P VKAGVETT TPSKQSNNKY AASSYLSLTP EQWKSHRSYS CQVTHEGSTV EKTVAPTECS |
-Macromolecule #2: Heavy Chain
| Macromolecule | Name: Heavy Chain / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.785742 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLVESGGN LAKPGGSLRL SCAASGFTFS DAWMTWVRQA PGKGLEWVGR IKSNADGGAT EYAAPVRGRF TISRDDSRSS LFLQMNSLK TEDTAVYYCL TPPPRRYWGQ GILVTVSSGS TKGPSVFPLA PSSKSTSGGT AALGCLVKDY FPEPVTVSWN S GALTSGVH ...String: EVQLVESGGN LAKPGGSLRL SCAASGFTFS DAWMTWVRQA PGKGLEWVGR IKSNADGGAT EYAAPVRGRF TISRDDSRSS LFLQMNSLK TEDTAVYYCL TPPPRRYWGQ GILVTVSSGS TKGPSVFPLA PSSKSTSGGT AALGCLVKDY FPEPVTVSWN S GALTSGVH TFPAVLQSSG LYSLSSVVTV PSSSLGTQTY ICNVNHKPSN TKVDKRVEPK SCDKTH |
-Macromolecule #3: Enveloped Glycoprotein 1
| Macromolecule | Name: Enveloped Glycoprotein 1 / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 35.706977 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ETGRSIPLGV IHNSALQVSD VDKLVCRDKL SSTNQLRSVG LNLEGNGVAT DVPSATKRWG FRSGVPPKVV NYEAGEWAEN CYNLEIKKP DGSECLPAAP DGIRGFPRCR YVHKVSGTGP CAGDFAFHKE GAFFLYDRLA STVIYRGTTF AEGVVAFLIL P QAKKDFFS ...String: ETGRSIPLGV IHNSALQVSD VDKLVCRDKL SSTNQLRSVG LNLEGNGVAT DVPSATKRWG FRSGVPPKVV NYEAGEWAEN CYNLEIKKP DGSECLPAAP DGIRGFPRCR YVHKVSGTGP CAGDFAFHKE GAFFLYDRLA STVIYRGTTF AEGVVAFLIL P QAKKDFFS SHPLREPVNA TEDPSSGYYS TTIRYQATGF GTNETEYLFE VDNLTYVQLE SRFTPQFLLQ LNETIYTSGK RS NTTGKLI WKVNPEIDTT IGEWAFWETK KNLTRKIRSE ELSFTVVSTH HQDTGEESAS SGKLGLITNT IAGVAGLITG GRR TRR |
-Macromolecule #4: Envelope glycoprotein
| Macromolecule | Name: Envelope glycoprotein / type: protein_or_peptide / ID: 4 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 18.989391 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EAIVNAQPKC NPNLHYWTTQ DEGAAIGLAW IPYFGPAAEG IYIEGLMHNQ DGLICGLRQL ANETTQALQL FLRATTELRT FSILNRKAI DFLLQRWGGT CHILGPDCCI EPHDWTKNIT DKIDQIIHDF VDGSGYIPEA PRDGQAYVRK DGEWVLLSTF L GTHHHHHH UniProtKB: Envelope glycoprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average exposure time: 27.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)