+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-0947 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Low resolution architecture of curli complex | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | curli / TRANSPORT PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報curli secretion complex / curli assembly / protein secretion by the type VIII secretion system / protein transmembrane transport / single-species biofilm formation / cell outer membrane / outer membrane-bounded periplasmic space / identical protein binding / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  | |||||||||
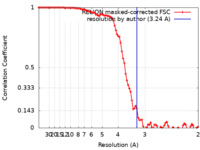
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.24 Å | |||||||||
 データ登録者 データ登録者 | Zhang M / Shi H | |||||||||
 引用 引用 |  ジャーナル: PLoS Biol / 年: 2020 ジャーナル: PLoS Biol / 年: 2020タイトル: Cryo-EM structure of the nonameric CsgG-CsgF complex and its implications for controlling curli biogenesis in Enterobacteriaceae. 著者: Manfeng Zhang / Huigang Shi / Xuemei Zhang / Xinzheng Zhang / Yihua Huang /  要旨: Curli play critical roles in biofilm formation, host cell adhesion, and colonization of inert surfaces in many Enterobacteriaceae. In Escherichia coli, curli biogenesis requires 7 curli-specific gene ...Curli play critical roles in biofilm formation, host cell adhesion, and colonization of inert surfaces in many Enterobacteriaceae. In Escherichia coli, curli biogenesis requires 7 curli-specific gene (csg) products-CsgA through G-working in concert. Of them, CsgG and CsgF are 2 outer membrane (OM)-localized components that consists of the core apparatus for secretion and assembly of curli structural subunits, CsgB and CsgA. Here, we report the cryogenic electron microscopy (cryo-EM) structure of CsgG in complex with CsgF from E. coli. The structure reveals that CsgF forms a stable complex with CsgG via a 1:1 stoichiometry by lining the upper lumen of the nonameric CsgG channel via its N-terminal 27 residues, forming a funnel-like entity plugged in the CsgG channel and creating a unique secretion channel with 2 constriction regions, consistent with the recently reported structure of the CsgG-CsgF complex. Functional studies indicate that export of CsgF to the cell surface requires the CsgG channel, and CsgF not only functions as an adaptor that bridges CsgB with CsgG but also may play important roles in controlling the rates of translocation and/or polymerization for curli structural subunits. Importantly, we found that a series of CsgF-derived peptides are able to efficiently inhibit curli production to E. coli when administrated exogenously, highlighting a potential strategy to interfere biofilm formation in E. coli strains. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_0947.map.gz emd_0947.map.gz | 59.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-0947-v30.xml emd-0947-v30.xml emd-0947.xml emd-0947.xml | 20.6 KB 20.6 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_0947_fsc.xml emd_0947_fsc.xml | 9.1 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_0947.png emd_0947.png | 51 KB | ||
| Filedesc metadata |  emd-0947.cif.gz emd-0947.cif.gz | 6.1 KB | ||
| その他 |  emd_0947_half_map_1.map.gz emd_0947_half_map_1.map.gz emd_0947_half_map_2.map.gz emd_0947_half_map_2.map.gz | 49.5 MB 49.4 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0947 http://ftp.pdbj.org/pub/emdb/structures/EMD-0947 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0947 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0947 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_0947_validation.pdf.gz emd_0947_validation.pdf.gz | 814.6 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_0947_full_validation.pdf.gz emd_0947_full_validation.pdf.gz | 814.2 KB | 表示 | |
| XML形式データ |  emd_0947_validation.xml.gz emd_0947_validation.xml.gz | 15 KB | 表示 | |
| CIF形式データ |  emd_0947_validation.cif.gz emd_0947_validation.cif.gz | 20.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0947 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0947 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0947 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0947 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_0947.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_0947.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-ハーフマップ: #2
| ファイル | emd_0947_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_0947_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : curli core complex of CsgG and CsgF
| 全体 | 名称: curli core complex of CsgG and CsgF |
|---|---|
| 要素 |
|
-超分子 #1: curli core complex of CsgG and CsgF
| 超分子 | 名称: curli core complex of CsgG and CsgF / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Curli production assembly/transport component CsgG
| 分子 | 名称: Curli production assembly/transport component CsgG / タイプ: protein_or_peptide / ID: 1 / コピー数: 9 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 31.62618 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MQRLFLLVAV MLLSGCLTAP PKEAARPTLM PRAQSYKDLT HLPAPTGKIF VSVYNIQDET GQFKPYPASN FSTAVPQSAT AMLVTALKD SRWFIPLERQ GLQNLLNERK IIRAAQENGT VAINNRIPLQ SLTAANIMVE GSIIGYESNV KSGGVGARYF G IGADTQYQ ...文字列: MQRLFLLVAV MLLSGCLTAP PKEAARPTLM PRAQSYKDLT HLPAPTGKIF VSVYNIQDET GQFKPYPASN FSTAVPQSAT AMLVTALKD SRWFIPLERQ GLQNLLNERK IIRAAQENGT VAINNRIPLQ SLTAANIMVE GSIIGYESNV KSGGVGARYF G IGADTQYQ LDQIAVNLRV VNVSTGEILS SVNTSKTILS YEVQAGVFRF IDYQRLLEGE VGYTSNEPVM LCLMSAIETG VI FLINDGI DRGLWDLQNK AERQNDILVK YRHMSVPPES WSHPQFEK UniProtKB: Curli production assembly/transport component CsgG |
-分子 #2: Curli production assembly/transport component CsgF
| 分子 | 名称: Curli production assembly/transport component CsgF / タイプ: protein_or_peptide / ID: 2 / コピー数: 9 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 15.894586 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MRVKHAVVLL MLISPLSWAG TMTFQFRNPN FGGNPNNGAF LLNSAQAQNS YKDPSYNDDF GIETPSALDN FTQAIQSQIL GGLLSNINT GKPGRMVTND YIVDIANRDG QLQLNVTDRK TGQTSTIQVS GLQNNSTDFH HHHHH UniProtKB: Curli production assembly/transport component CsgF |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| グリッド | 材質: COPPER / メッシュ: 300 / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 30 sec. / 前処理 - 雰囲気: AIR |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 80 % / チャンバー内温度: 298 K |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI ARCTICA |
|---|---|
| 温度 | 最低: 70.0 K |
| アライメント法 | Coma free - Residual tilt: 0.1 mrad |
| 特殊光学系 | エネルギーフィルター - 名称: GIF Bioquantum / エネルギーフィルター - スリット幅: 20 eV |
| 撮影 | フィルム・検出器のモデル: GATAN K2 BASE (4k x 4k) デジタル化 - サイズ - 横: 3710 pixel / デジタル化 - サイズ - 縦: 3838 pixel / 撮影したグリッド数: 1 / 実像数: 2500 / 平均電子線量: 48.0 e/Å2 |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 70.0 µm / 最大 デフォーカス(補正後): 2.5 µm / 最小 デフォーカス(補正後): 1.0 µm / 照射モード: SPOT SCAN / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 1.8 µm / 最小 デフォーカス(公称値): 1.2 µm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Talos Arctica / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)