+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0780 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryo-EM structure of human PA200 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Proteasome activator / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationspermatoproteasome complex / sperm DNA condensation / Proteasome assembly / peptidase activator activity / proteasomal ubiquitin-independent protein catabolic process / proteasome binding / : / nuclear speck / DNA repair / DNA damage response ...spermatoproteasome complex / sperm DNA condensation / Proteasome assembly / peptidase activator activity / proteasomal ubiquitin-independent protein catabolic process / proteasome binding / : / nuclear speck / DNA repair / DNA damage response / nucleoplasm / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.75 Å | |||||||||
 Authors Authors | Ouyang S / Hongxin G | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2020 Journal: PLoS Biol / Year: 2020Title: Cryo-EM structures of the human PA200 and PA200-20S complex reveal regulation of proteasome gate opening and two PA200 apertures. Authors: Hongxin Guan / Youwang Wang / Ting Yu / Yini Huang / Mianhuan Li / Abdullah F U H Saeed / Vanja Perčulija / Daliang Li / Jia Xiao / Dongmei Wang / Ping Zhu / Songying Ouyang /  Abstract: Proteasomes are highly abundant and conserved protease complexes that eliminate unwanted proteins in the cells. As a single-chain ATP-independent nuclear proteasome activator, proteasome activator ...Proteasomes are highly abundant and conserved protease complexes that eliminate unwanted proteins in the cells. As a single-chain ATP-independent nuclear proteasome activator, proteasome activator 200 (PA200) associates with 20S core particle to form proteasome complex that catalyzes polyubiquitin-independent degradation of acetylated histones, thus playing a pivotal role in DNA repair and spermatogenesis. Here, we present cryo-electron microscopy (cryo-EM) structures of the human PA200-20S complex and PA200 at 2.72 Å and 3.75 Å, respectively. PA200 exhibits a dome-like architecture that caps 20S and uses its C-terminal YYA (Tyr-Tyr-Ala) to induce the α-ring rearrangements and partial opening of the 20S gate. Our structural data also indicate that PA200 has two openings formed by numerous positively charged residues that respectively bind (5,6)-bisdiphosphoinositol tetrakisphosphate (5,6[PP]2-InsP4) and inositol hexakisphosphate (InsP6) and are likely to be the gates that lead unfolded proteins through PA200 and into the 20S. Besides, our structural analysis of PA200 found that the bromodomain (BRD)-like (BRDL) domain of PA200 shows considerable sequence variation in comparison to other human BRDs, as it contains only 82 residues because of a short ZA loop, and cannot be classified into any of the eight typical human BRD families. Taken together, the results obtained from this study provide important insights into human PA200-induced 20S gate opening for substrate degradation and the opportunities to explore the mechanism for its recognition of H4 histone in acetylation-mediated proteasomal degradation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0780.map.gz emd_0780.map.gz | 28.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0780-v30.xml emd-0780-v30.xml emd-0780.xml emd-0780.xml | 11.5 KB 11.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0780_fsc.xml emd_0780_fsc.xml | 7 KB | Display |  FSC data file FSC data file |
| Images |  emd_0780.png emd_0780.png | 35.6 KB | ||
| Filedesc metadata |  emd-0780.cif.gz emd-0780.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0780 http://ftp.pdbj.org/pub/emdb/structures/EMD-0780 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0780 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0780 | HTTPS FTP |
-Related structure data
| Related structure data |  6kwxMC  0781C  6kwyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0780.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0780.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human PA200
| Entire | Name: human PA200 |
|---|---|
| Components |
|
-Supramolecule #1: human PA200
| Supramolecule | Name: human PA200 / type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Proteasome activator complex subunit 4
| Macromolecule | Name: Proteasome activator complex subunit 4 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 215.774875 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGTTRSTMSY YHHHHHHDYD IPTTENLYFQ GAMDPMEPAE RAGVGEPPEP GGRPEPGPRG FVPQKEIVYN KLLPYAERLD AESDLQLAQ IKCNLGRAVQ LQELWPGGLF WTRKLSTYIR LYGRKFSKED HVLFIKLLYE LVSIPKLEIS MMQGFARLLI N LLKKKELL ...String: MGTTRSTMSY YHHHHHHDYD IPTTENLYFQ GAMDPMEPAE RAGVGEPPEP GGRPEPGPRG FVPQKEIVYN KLLPYAERLD AESDLQLAQ IKCNLGRAVQ LQELWPGGLF WTRKLSTYIR LYGRKFSKED HVLFIKLLYE LVSIPKLEIS MMQGFARLLI N LLKKKELL SRADLELPWR PLYDMVERIL YSKTEHLGLN WFPNSVENIL KTLVKSCRPY FPADATAEML EEWRPLMCPF DV TMQKAIT YFEIFLPTSL PPELHHKGFK LWFDELIGLW VSVQNLPQWE GQLVNLFARL ATDNIGYIDW DPYVPKIFTR ILR SLNLPV GSSQVLVPRF LTNAYDIGHA VIWITAMMGG PSKLVQKHLA GLFNSITSFY HPSNNGRWLN KLMKLLQRLP NSVV RRLHR ERYKKPSWLT PVPDSHKLTD QDVTDFVQCI IQPVLLAMFS KTGSLEAAQA LQNLALMRPE LVIPPVLERT YPALE TLTE PHQLTATLSC VIGVARSLVS GGRWFPEGPT HMLPLLMRAL PGVDPNDFSK CMITFQFIAT FSTLVPLVDC SSVLQE RND LTEVERELCS ATAEFEDFVL QFMDRCFGLI ESSTLEQTRE ETETEKMTHL ESLVELGLSS TFSTILTQCS KEIFMVA LQ KVFNFSTSHI FETRVAGRMV ADMCRAAVKC CPEESLKLFV PHCCSVITQL TMNDDVLNDE ELDKELLWNL QLLSEITR V DGRKLLLYRE QLVKILQRTL HLTCKQGYTL SCNLLHHLLR STTLIYPTEY CSVPGGFDKP PSEYFPIKDW GKPGDLWNL GIQWHVPSSE EVSFAFYLLD SFLQPELVKL QHCGDGKLEM SRDDILQSLT IVHNCILGSG NLLPPLKGEP VTNLVPSMVS LEETKLYTG LEYDLSRENH REVIATVIRK LLNHILDNSE DDTKSLFLII KIIGDLLQFQ GSHKHEFDSR WKSFNLVKKS M ENRLHGKK QHIRALLIDR VMLQHELRTL TVEGCEYKKI HQDMIRDLLR LSTSSYSQVR NKAQQTFFAA LGAYNFCCRD II PLVLEFL RPDRQGVTQQ QFKGALYCLL GNHSGVCLAN LHDWDCIVQT WPAIVSSGLS QAMSLEKPSI VRLFDDLAEK IHR QYETIG LDFTIPKSCV EIAELLQQSK NPSINQILLS PEKIKEGIKR QQEKNADALR NYENLVDTLL DGVEQRNLPW KFEH IGIGL LSLLLRDDRV LPLRAIRFFV ENLNHDAIVV RKMAISAVAG ILKQLKRTHK KLTINPCEIS GCPKPTQIIA GDRPD NHWL HYDSKTIPRT KKEWESSCFV EKTHWGYYTW PKNMVVYAGV EEQPKLGRSR EDMTEAEQII FDHFSDPKFV EQLITF LSL EDRKGKDKFN PRRFCLFKGI FRNFDDAFLP VLKPHLEHLV ADSHESTQRC VAEIIAGLIR GSKHWTFEKV EKLWELL CP LLRTALSNIT VETYNDWGAC IATSCESRDP RKLHWLFELL LESPLSGEGG SFVDACRLYV LQGGLAQQEW RVPELLHR L LKYLEPKLTQ VYKNVRERIG SVLTYIFMID VSLPNTTPTI SPHVPEFTAR ILEKLKPLMD VDEEIQNHVM EENGIGEED ERTQGIKLLK TILKWLMASA GRSFSTAVTE QLQLLPLFFK IAPVENDNSY DELKRDAKLC LSLMSQGLLY PHQVPLVLQV LKQTARSSS WHARYTVLTY LQTMVFYNLF IFLNNEDAVK DIRWLVISLL EDEQLEVREM AATTLSGLLQ CNFLTMDSPM Q IHFEQLCK TKLPKKRKRD PGSVGDTIPS AELVKRHAGV LGLGACVLSS PYDVPTWMPQ LLMNLSAHLN DPQPIEMTVK KT LSNFRRT HHDNWQEHKQ QFTDDQLLVL TDLLVSPCYY A UniProtKB: Proteasome activator complex subunit 4 |
-Macromolecule #2: [(1~{S},2~{R},3~{R},4~{S},5~{S},6~{R})-2-[oxidanyl(phosphonooxy)p...
| Macromolecule | Name: [(1~{S},2~{R},3~{R},4~{S},5~{S},6~{R})-2-[oxidanyl(phosphonooxy)phosphoryl]oxy-3,4,5,6-tetraphosphonooxy-cyclohexyl] phosphono hydrogen phosphate type: ligand / ID: 2 / Number of copies: 1 / Formula: K0W |
|---|---|
| Molecular weight | Theoretical: 819.995 Da |
| Chemical component information | 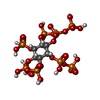 ChemComp-K0W: |
-Macromolecule #3: INOSITOL HEXAKISPHOSPHATE
| Macromolecule | Name: INOSITOL HEXAKISPHOSPHATE / type: ligand / ID: 3 / Number of copies: 1 / Formula: IHP |
|---|---|
| Molecular weight | Theoretical: 660.035 Da |
| Chemical component information |  ChemComp-IHP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















