+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0336 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the yeast Sec complex | |||||||||
 Map data Map data | Sharpened full map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Sec61 / Sec63 / Sec71 / Sec72 / Sec66 / protein translocation / translocon / endoplasmic reticulum / secretion / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmisfolded protein transport / Sec62/Sec63 complex / translocon complex / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / rough endoplasmic reticulum membrane / cytosol to endoplasmic reticulum transport / Ssh1 translocon complex / Sec61 translocon complex / protein-transporting ATPase activity / post-translational protein targeting to endoplasmic reticulum membrane ...misfolded protein transport / Sec62/Sec63 complex / translocon complex / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / rough endoplasmic reticulum membrane / cytosol to endoplasmic reticulum transport / Ssh1 translocon complex / Sec61 translocon complex / protein-transporting ATPase activity / post-translational protein targeting to endoplasmic reticulum membrane / SRP-dependent cotranslational protein targeting to membrane, translocation / filamentous growth / signal sequence binding / SRP-dependent cotranslational protein targeting to membrane / post-translational protein targeting to membrane, translocation / peptide transmembrane transporter activity / nuclear inner membrane / retrograde protein transport, ER to cytosol / protein transmembrane transporter activity / ERAD pathway / guanyl-nucleotide exchange factor activity / cell periphery / ribosome binding / endoplasmic reticulum membrane / structural molecule activity / endoplasmic reticulum / mitochondrion / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
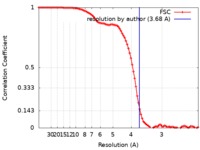
| Method | single particle reconstruction / cryo EM / Resolution: 3.68 Å | |||||||||
 Authors Authors | Park E / Itskanov S | |||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Structure of the posttranslational Sec protein-translocation channel complex from yeast. Authors: Samuel Itskanov / Eunyong Park /  Abstract: The Sec61 protein-conducting channel mediates transport of many proteins, such as secretory proteins, across the endoplasmic reticulum (ER) membrane during or after translation. Posttranslational ...The Sec61 protein-conducting channel mediates transport of many proteins, such as secretory proteins, across the endoplasmic reticulum (ER) membrane during or after translation. Posttranslational transport is enabled by two additional membrane proteins associated with the channel, Sec63 and Sec62, but its mechanism is poorly understood. We determined a structure of the Sec complex (Sec61-Sec63-Sec71-Sec72) from by cryo-electron microscopy (cryo-EM). The structure shows that Sec63 tightly associates with Sec61 through interactions in cytosolic, transmembrane, and ER-luminal domains, prying open Sec61's lateral gate and translocation pore and thus activating the channel for substrate engagement. Furthermore, Sec63 optimally positions binding sites for cytosolic and luminal chaperones in the complex to enable efficient polypeptide translocation. Our study provides mechanistic insights into eukaryotic posttranslational protein translocation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0336.map.gz emd_0336.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0336-v30.xml emd-0336-v30.xml emd-0336.xml emd-0336.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0336_fsc.xml emd_0336_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_0336.png emd_0336.png | 58 KB | ||
| Filedesc metadata |  emd-0336.cif.gz emd-0336.cif.gz | 6.7 KB | ||
| Others |  emd_0336_additional.map.gz emd_0336_additional.map.gz | 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0336 http://ftp.pdbj.org/pub/emdb/structures/EMD-0336 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0336 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0336 | HTTPS FTP |
-Related structure data
| Related structure data |  6n3qMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0336.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0336.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened full map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unfiltered full map
| File | emd_0336_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered full map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Posttranslational Sec protein-translocation channel complex
| Entire | Name: Posttranslational Sec protein-translocation channel complex |
|---|---|
| Components |
|
-Supramolecule #1: Posttranslational Sec protein-translocation channel complex
| Supramolecule | Name: Posttranslational Sec protein-translocation channel complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Protein transport protein SEC61
| Macromolecule | Name: Protein transport protein SEC61 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 52.978148 KDa |
| Sequence | String: MSSNRVLDLF KPFESFLPEV IAPERKVPYN QKLIWTGVSL LIFLILGQIP LYGIVSSETS DPLYWLRAML ASNRGTLLEL GVSPIITSS MIFQFLQGTQ LLQIRPESKQ DRELFQIAQK VCAIILILGQ ALVVVMTGNY GAPSDLGLPI CLLLIFQLMF A SLIVMLLD ...String: MSSNRVLDLF KPFESFLPEV IAPERKVPYN QKLIWTGVSL LIFLILGQIP LYGIVSSETS DPLYWLRAML ASNRGTLLEL GVSPIITSS MIFQFLQGTQ LLQIRPESKQ DRELFQIAQK VCAIILILGQ ALVVVMTGNY GAPSDLGLPI CLLLIFQLMF A SLIVMLLD ELLSKGYGLG SGISLFTATN IAEQIFWRAF APTTVNSGRG KEFEGAVIAF FHLLAVRKDK KRALVEAFYR TN LPNMFQV LMTVAIFLFV LYLQGFRYEL PIRSTKVRGQ IGIYPIKLFY TSNTPIMLQS ALTSNIFLIS QILFQKYPTN PLI RLIGVW GIRPGTQGPQ MALSGLAYYI QPLMSLSEAL LDPIKTIVYI TFVLGSCAVF SKTWIEISGT SPRDIAKQFK DQGM VINGK RETSIYRELK KIIPTAAAFG GATIGALSVG SDLLGTLGSG ASILMATTTI YGYYEAAAKE GGFTKNLVPG FSDLM UniProtKB: Protein transport protein SEC61 |
-Macromolecule #2: Protein transport protein SSS1
| Macromolecule | Name: Protein transport protein SSS1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 8.958641 KDa |
| Sequence | String: MARASEKGEE KKQSNNQVEK LVEAPVEFVR EGTQFLAKCK KPDLKEYTKI VKAVGIGFIA VGIIGYAIKL IHIPIRYVIV UniProtKB: Protein transport protein SSS1 |
-Macromolecule #3: Protein transport protein SBH1
| Macromolecule | Name: Protein transport protein SBH1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 8.723155 KDa |
| Sequence | String: MSSPTPPGGQ RTLQKRKQGS SQKVAASAPK KNTNSNNSIL KIYSDEATGL RVDPLVVLFL AVGFIFSVVA LHVISKVAGK LF UniProtKB: Protein transport protein SBH1 |
-Macromolecule #4: Protein translocation protein SEC63
| Macromolecule | Name: Protein translocation protein SEC63 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 75.432258 KDa |
| Sequence | String: MPTNYEYDEA SETWPSFILT GLLMVVGPMT LLQIYQIFFG ANAEDGNSGK SKEFNEEVFK NLNEEYTSDE IKQFRRKFDK NSNKKSKIW SRRNIIIIVG WILVAILLQR INSNDAIKDA ATKLFDPYEI LGISTSASDR DIKSAYRKLS VKFHPDKLAK G LTPDEKSV ...String: MPTNYEYDEA SETWPSFILT GLLMVVGPMT LLQIYQIFFG ANAEDGNSGK SKEFNEEVFK NLNEEYTSDE IKQFRRKFDK NSNKKSKIW SRRNIIIIVG WILVAILLQR INSNDAIKDA ATKLFDPYEI LGISTSASDR DIKSAYRKLS VKFHPDKLAK G LTPDEKSV MEETYVQITK AYESLTDELV RQNYLKYGHP DGPQSTSHGI ALPRFLVDGS ASPLLVVCYV ALLGLILPYF VS RWWARTQ SYTKKGIHNV TASNFVSNLV NYKPSEIVTT DLILHWLSFA HEFKQFFPDL QPTDFEKLLQ DHINRRDSGK LNN AKFRIV AKCHSLLHGL LDIACGFRNL DIALGAINTF KCIVQAVPLT PNCQILQLPN VDKEHFITKT GDIHTLGKLF TLED AKIGE VLGIKDQAKL NETLRVASHI PNLKIIKADF LVPGENQVTP SSTPYISLKV LVRSAKQPLI PTSLIPEENL TEPQD FESQ RDPFAMMSKQ PLVPYSFAPF FPTKRRGSWC CLVSSQKDGK ILQTPIIIEK LSYKNLNDDK DFFDKRIKMD LTKHEK FDI NDWEIGTIKI PLGQPAPETV GDFFFRVIVK STDYFTTDLD ITMNMKVRDS PAVEQVEVYS EEDDEYSTDD DETESDD ES DASDYTDIDT DTEAEDDESP E UniProtKB: Protein translocation protein SEC63 |
-Macromolecule #5: Translocation protein SEC66
| Macromolecule | Name: Translocation protein SEC66 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 24.263939 KDa |
| Sequence | String: MSEFNETKFS NNGTFFETEE PIVETKSISV YTPLIYVFIL VVSLVMFASS YRKKQAKKIS EQPSIFDEND AHDLYFQIKE MSENEKIHE KVLKAALLNR GAESVRRSLK LKELAPQINL LYKNGSIGED YWKRFETEVK LIELEFKDTL QEAERLQPGW V QLFVMVCK ...String: MSEFNETKFS NNGTFFETEE PIVETKSISV YTPLIYVFIL VVSLVMFASS YRKKQAKKIS EQPSIFDEND AHDLYFQIKE MSENEKIHE KVLKAALLNR GAESVRRSLK LKELAPQINL LYKNGSIGED YWKRFETEVK LIELEFKDTL QEAERLQPGW V QLFVMVCK EICFNQALSR RYQSILKRKE VCIKEWELKI NNDGRLVN UniProtKB: Translocation protein SEC66 |
-Macromolecule #6: Translocation protein SEC72
| Macromolecule | Name: Translocation protein SEC72 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 21.63109 KDa |
| Sequence | String: MVTLEYNANS KLITASDAVV ALSTETNIDQ INVLTTSLIG ETNPNFTPQP NEALSKMIKG LFESGMKNLQ QKKLNEALKN VSLAIEMAQ RKRAPWEAFA IQLPELHFML RSKIDLCLIL GKHLEALQDL DFLLGTGLIQ PDVFVRKADC LLKLRQWEEA R ATCERGLA ...String: MVTLEYNANS KLITASDAVV ALSTETNIDQ INVLTTSLIG ETNPNFTPQP NEALSKMIKG LFESGMKNLQ QKKLNEALKN VSLAIEMAQ RKRAPWEAFA IQLPELHFML RSKIDLCLIL GKHLEALQDL DFLLGTGLIQ PDVFVRKADC LLKLRQWEEA R ATCERGLA LAPEDMKLRA LLIETARNLA EYNGE UniProtKB: Translocation protein SEC72 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average exposure time: 8.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 43103 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)