[English] 日本語
 Yorodumi
Yorodumi- EMDB-0159: Retromer-Vps5: map centred on the Vps5 layer under the Vps26 dimer. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0159 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Retromer-Vps5: map centred on the Vps5 layer under the Vps26 dimer. | |||||||||
 Map data Map data | Retromer-Vps5: map centred on the Vps5 layer under the Vps26 dimer. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Chaetomium thermophilum (fungus) Chaetomium thermophilum (fungus) | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 8.9 Å | |||||||||
 Authors Authors | Kovtun O / Leneva N / Ariotti N / Teasdale RD / Owen DJ / Collins BM / Briggs JAG | |||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Authors: Oleksiy Kovtun / Natalya Leneva / Yury S Bykov / Nicholas Ariotti / Rohan D Teasdale / Miroslava Schaffer / Benjamin D Engel / David J Owen / John A G Briggs / Brett M Collins /    Abstract: Eukaryotic cells traffic proteins and lipids between different compartments using protein-coated vesicles and tubules. The retromer complex is required to generate cargo-selective tubulovesicular ...Eukaryotic cells traffic proteins and lipids between different compartments using protein-coated vesicles and tubules. The retromer complex is required to generate cargo-selective tubulovesicular carriers from endosomal membranes. Conserved in eukaryotes, retromer controls the cellular localization and homeostasis of hundreds of transmembrane proteins, and its disruption is associated with major neurodegenerative disorders. How retromer is assembled and how it is recruited to form coated tubules is not known. Here we describe the structure of the retromer complex (Vps26-Vps29-Vps35) assembled on membrane tubules with the bin/amphiphysin/rvs-domain-containing sorting nexin protein Vps5, using cryo-electron tomography and subtomogram averaging. This reveals a membrane-associated Vps5 array, from which arches of retromer extend away from the membrane surface. Vps35 forms the 'legs' of these arches, and Vps29 resides at the apex where it is free to interact with regulatory factors. The bases of the arches connect to each other and to Vps5 through Vps26, and the presence of the same arches on coated tubules within cells confirms their functional importance. Vps5 binds to Vps26 at a position analogous to the previously described cargo- and Snx3-binding site, which suggests the existence of distinct retromer-sorting nexin assemblies. The structure provides insight into the architecture of the coat and its mechanism of assembly, and suggests that retromer promotes tubule formation by directing the distribution of sorting nexin proteins on the membrane surface while providing a scaffold for regulatory-protein interactions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0159.map.gz emd_0159.map.gz | 20.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0159-v30.xml emd-0159-v30.xml emd-0159.xml emd-0159.xml | 13.6 KB 13.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0159.png emd_0159.png | 45.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0159 http://ftp.pdbj.org/pub/emdb/structures/EMD-0159 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0159 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0159 | HTTPS FTP |
-Related structure data
| Related structure data |  0154C  0155C  0156C  0157C  0158C  0160C  0161C  0162C  0163C  5w8mC  6h7wC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0159.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0159.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Retromer-Vps5: map centred on the Vps5 layer under the Vps26 dimer. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
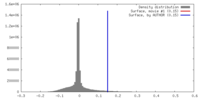
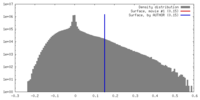
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Retromer-Vps5 complex assembled on the membrane.
| Entire | Name: Retromer-Vps5 complex assembled on the membrane. |
|---|---|
| Components |
|
-Supramolecule #1: Retromer-Vps5 complex assembled on the membrane.
| Supramolecule | Name: Retromer-Vps5 complex assembled on the membrane. / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Vps26/Vps35/Vps29 trimer recruited to the membrane via Vps5 PX-BAR protein. |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (fungus) Chaetomium thermophilum (fungus) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Vacuolar protein sorting-associated protein 5
| Macromolecule | Name: Vacuolar protein sorting-associated protein 5 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MEDPWADTAS PNSSAPLSQH VEDSSSAPAA AAETPSNTAT TTAPSISTSS RPSRATPRRI VAKPKSLEAV EDDPLGPLGA PTPSSDSDNN GLGGATARGV QPPVPPLKEQ LPLRTTLPPS TTNRGGRGRR SGPPDPHHID DDDDDAALFG SAGRGRPPPP VPASLPSPVK ...String: MEDPWADTAS PNSSAPLSQH VEDSSSAPAA AAETPSNTAT TTAPSISTSS RPSRATPRRI VAKPKSLEAV EDDPLGPLGA PTPSSDSDNN GLGGATARGV QPPVPPLKEQ LPLRTTLPPS TTNRGGRGRR SGPPDPHHID DDDDDAALFG SAGRGRPPPP VPASLPSPVK SSTAPSMSVE QAARPTFHIT VGDPHKVGDL ATSHIVYSVR TKTTSKAYKQ PEFEVKRRYR DFLWLYNTLH SNNPGVVVPP PPEKQAVGRF ESNFVESRRA ALEKMLNKIA AHPTLQLDAD LKLFLESESF NIDVKHKERK EPPLGESKGV FGSLGFGGGG NKFVEQDDWF HDRRVYLDAL ENQLKALLKA MDNMVAQRKA MAEAAADFSA SLHALSTVEL SPTLSGPLDA LSELQLAIRD VYERQAQQDV LTFGIIIEEY IRLIGSVKQA FSQRQKAFHS WHSAESELMK KKAAQDKLLR QGKTQQDRLN QVNAEVIDAE RKVHQARLLF EDMGRLLRSE LDRFEREKVE DFKSGVETFL ESAVEAQKEL IEKWETFLMQ LDAEDDESVF YRPPPVQTKK PAGDTAVDRA RARIDEDSD |
-Macromolecule #2: Vacuolar protein sorting-associated protein 26
| Macromolecule | Name: Vacuolar protein sorting-associated protein 26 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MSYFFSTPVD IDIVLADADK RAMVDVKLDK NRREKVPLYM DGESVKGCVT VRPKDGKRLE HTGIKVQFIG TIEMFFDRGN HYEFLSLVQE LAAPGELQHP QTFDFNFKNV EKQYESYNGI NVKLRYFVRV TVSRRMADVI REKDIWVYSY RIPPELNSSI KMDVGIEDCL ...String: MSYFFSTPVD IDIVLADADK RAMVDVKLDK NRREKVPLYM DGESVKGCVT VRPKDGKRLE HTGIKVQFIG TIEMFFDRGN HYEFLSLVQE LAAPGELQHP QTFDFNFKNV EKQYESYNGI NVKLRYFVRV TVSRRMADVI REKDIWVYSY RIPPELNSSI KMDVGIEDCL HIEFEYSKSK YHLKDVIVGR IYFLLVRLKI KHMELSIIRR ETTGVAPNQY NESETLVRFE IMDGSPSRGE TIPIRLFLGG FDLTPTFRDV NKKFSTRYYL SLVLIDEDAR RYFKQSEIIL YRQPPEYDNQ LLPPPPDQKQ VTAPLA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Concentration | 1.1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 292 K / Instrument: LEICA EM GP | |||||||||
| Details | The solution-assembled complex was incubated with Folch liposomes at room temperature. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 2 / Average electron dose: 3.17 e/Å2 Details: Tomographic tilt series were acquired with the dose-symmetric tilt-scheme (Hagen et al., J Struct Biol. 2017) |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 6.5 µm / Nominal defocus min: 2.5 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)