[English] 日本語
 Yorodumi
Yorodumi- PDB-8uxg: Structure of PKA phosphorylated human RyR2-R420W in the closed st... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8uxg | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of PKA phosphorylated human RyR2-R420W in the closed state in the presence of ARM210 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSPORT PROTEIN / calcium channel | ||||||
| Function / homology |  Function and homology information Function and homology informationsarcoplasmic reticulum calcium ion transport / establishment of protein localization to endoplasmic reticulum / junctional sarcoplasmic reticulum membrane / type B pancreatic cell apoptotic process / Purkinje myocyte to ventricular cardiac muscle cell signaling / regulation of atrial cardiac muscle cell action potential / calcium-induced calcium release activity / left ventricular cardiac muscle tissue morphogenesis / suramin binding / regulation of AV node cell action potential ...sarcoplasmic reticulum calcium ion transport / establishment of protein localization to endoplasmic reticulum / junctional sarcoplasmic reticulum membrane / type B pancreatic cell apoptotic process / Purkinje myocyte to ventricular cardiac muscle cell signaling / regulation of atrial cardiac muscle cell action potential / calcium-induced calcium release activity / left ventricular cardiac muscle tissue morphogenesis / suramin binding / regulation of AV node cell action potential / regulation of SA node cell action potential / cell communication by electrical coupling involved in cardiac conduction / regulation of ventricular cardiac muscle cell action potential / ventricular cardiac muscle cell action potential / positive regulation of sequestering of calcium ion / negative regulation of calcium-mediated signaling / embryonic heart tube morphogenesis / cardiac muscle hypertrophy / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / calcium ion transport into cytosol / insulin secretion involved in cellular response to glucose stimulus / ryanodine-sensitive calcium-release channel activity / response to caffeine / regulation of cardiac muscle contraction by calcium ion signaling / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to redox state / negative regulation of heart rate / cellular response to caffeine / 'de novo' protein folding / FK506 binding / response to muscle activity / protein kinase A catalytic subunit binding / protein kinase A regulatory subunit binding / positive regulation of the force of heart contraction / intracellularly gated calcium channel activity / smooth endoplasmic reticulum / smooth muscle contraction / detection of calcium ion / regulation of cytosolic calcium ion concentration / regulation of cardiac muscle contraction / T cell proliferation / positive regulation of heart rate / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / calcium channel inhibitor activity / cardiac muscle contraction / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / Ion homeostasis / response to muscle stretch / release of sequestered calcium ion into cytosol / cellular response to epinephrine stimulus / calcium channel complex / sarcoplasmic reticulum membrane / regulation of heart rate / sarcoplasmic reticulum / protein maturation / calcium channel regulator activity / peptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / establishment of localization in cell / calcium-mediated signaling / sarcolemma / Stimuli-sensing channels / calcium channel activity / Z disc / intracellular calcium ion homeostasis / calcium ion transport / positive regulation of cytosolic calcium ion concentration / protein refolding / transmembrane transporter binding / response to hypoxia / calmodulin binding / signaling receptor binding / calcium ion binding / enzyme binding / protein-containing complex / identical protein binding / membrane / plasma membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.08 Å | ||||||
 Authors Authors | Miotto, M.C. / Marks, A.R. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis for ryanodine receptor type 2 leak in heart failure and arrhythmogenic disorders. Authors: Marco C Miotto / Steven Reiken / Anetta Wronska / Qi Yuan / Haikel Dridi / Yang Liu / Gunnar Weninger / Carl Tchagou / Andrew R Marks /  Abstract: Heart failure, the leading cause of mortality and morbidity in the developed world, is characterized by cardiac ryanodine receptor 2 channels that are hyperphosphorylated, oxidized, and depleted of ...Heart failure, the leading cause of mortality and morbidity in the developed world, is characterized by cardiac ryanodine receptor 2 channels that are hyperphosphorylated, oxidized, and depleted of the stabilizing subunit calstabin-2. This results in a diastolic sarcoplasmic reticulum Ca leak that impairs cardiac contractility and triggers arrhythmias. Genetic mutations in ryanodine receptor 2 can also cause Ca leak, leading to arrhythmias and sudden cardiac death. Here, we solved the cryogenic electron microscopy structures of ryanodine receptor 2 variants linked either to heart failure or inherited sudden cardiac death. All are in the primed state, part way between closed and open. Binding of Rycal drugs to ryanodine receptor 2 channels reverts the primed state back towards the closed state, decreasing Ca leak, improving cardiac function, and preventing arrhythmias. We propose a structural-physiological mechanism whereby the ryanodine receptor 2 channel primed state underlies the arrhythmias in heart failure and arrhythmogenic disorders. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8uxg.cif.gz 8uxg.cif.gz | 2.9 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8uxg.ent.gz pdb8uxg.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8uxg.json.gz 8uxg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8uxg_validation.pdf.gz 8uxg_validation.pdf.gz | 1.6 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8uxg_full_validation.pdf.gz 8uxg_full_validation.pdf.gz | 1.9 MB | Display | |
| Data in XML |  8uxg_validation.xml.gz 8uxg_validation.xml.gz | 406 KB | Display | |
| Data in CIF |  8uxg_validation.cif.gz 8uxg_validation.cif.gz | 625.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ux/8uxg https://data.pdbj.org/pub/pdb/validation_reports/ux/8uxg ftp://data.pdbj.org/pub/pdb/validation_reports/ux/8uxg ftp://data.pdbj.org/pub/pdb/validation_reports/ux/8uxg | HTTPS FTP |
-Related structure data
| Related structure data |  42763MC  8uq2C  8uq3C  8uq4C  8uq5C  8uxcC  8uxeC  8uxfC  8uxhC  8uxiC  8uxlC  8uxmC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
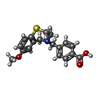
| #1: Protein | Mass: 565315.125 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: RYR2 / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Gene: RYR2 / Cell line (production host): HEK293 / Production host:  Homo sapiens (human) / References: UniProt: Q92736 Homo sapiens (human) / References: UniProt: Q92736#2: Protein | Mass: 11798.501 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: FKBP1B, FKBP12.6, FKBP1L, FKBP9, OTK4 / Production host: Homo sapiens (human) / Gene: FKBP1B, FKBP12.6, FKBP1L, FKBP9, OTK4 / Production host:  #3: Chemical | ChemComp-ZN / #4: Chemical | ChemComp-ATP / #5: Chemical | ChemComp-KVR / Has ligand of interest | Y | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| |||||||||||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| |||||||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | |||||||||||||||||||||||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 2.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1200 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 100 K / Temperature (min): 80 K |
| Image recording | Electron dose: 58 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV |
| Image scans | Sampling size: 5 µm / Width: 5760 / Height: 4092 |
- Processing
Processing
| EM software |
| |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | |||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C4 (4 fold cyclic) | |||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.08 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 181724 / Symmetry type: POINT | |||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 7UA5 Accession code: 7UA5 / Source name: PDB / Type: experimental model | |||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller









































































 PDBj
PDBj