[English] 日本語
 Yorodumi
Yorodumi- PDB-8fhs: Human L-type voltage-gated calcium channel Cav1.2 in the presence... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8fhs | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human L-type voltage-gated calcium channel Cav1.2 in the presence of amiodarone and sofosbuvir at 3.3 Angstrom resolution | |||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||
 Keywords Keywords | TRANSPORT PROTEIN / Cav1.2 / Channels / Calcium Ion-Selective | |||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationvoltage-gated calcium channel activity involved in AV node cell action potential / voltage-gated calcium channel activity involved in cardiac muscle cell action potential / positive regulation of high voltage-gated calcium channel activity / immune system development / positive regulation of adenylate cyclase activity / regulation of membrane repolarization during action potential / Presynaptic depolarization and calcium channel opening / membrane depolarization during atrial cardiac muscle cell action potential / Phase 2 - plateau phase / calcium ion transmembrane transport via high voltage-gated calcium channel ...voltage-gated calcium channel activity involved in AV node cell action potential / voltage-gated calcium channel activity involved in cardiac muscle cell action potential / positive regulation of high voltage-gated calcium channel activity / immune system development / positive regulation of adenylate cyclase activity / regulation of membrane repolarization during action potential / Presynaptic depolarization and calcium channel opening / membrane depolarization during atrial cardiac muscle cell action potential / Phase 2 - plateau phase / calcium ion transmembrane transport via high voltage-gated calcium channel / membrane depolarization during AV node cell action potential / high voltage-gated calcium channel activity / membrane depolarization during bundle of His cell action potential / cardiac conduction / L-type voltage-gated calcium channel complex / membrane depolarization during cardiac muscle cell action potential / positive regulation of muscle contraction / cell communication by electrical coupling involved in cardiac conduction / regulation of ventricular cardiac muscle cell action potential / regulation of ventricular cardiac muscle cell membrane repolarization / NCAM1 interactions / camera-type eye development / cardiac muscle cell action potential involved in contraction / embryonic forelimb morphogenesis / calcium ion transport into cytosol / regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / voltage-gated calcium channel complex / Mechanical load activates signaling by PIEZO1 and integrins in osteocytes / Phase 0 - rapid depolarisation / neuronal dense core vesicle / alpha-actinin binding / regulation of heart rate by cardiac conduction / regulation of calcium ion transport / calcium ion import across plasma membrane / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / voltage-gated calcium channel activity / presynaptic active zone membrane / calcium channel regulator activity / sarcoplasmic reticulum / protein localization to plasma membrane / Regulation of insulin secretion / postsynaptic density membrane / GABA-ergic synapse / calcium ion transmembrane transport / Z disc / cellular response to amyloid-beta / Adrenaline,noradrenaline inhibits insulin secretion / calcium ion transport / T cell receptor signaling pathway / heart development / positive regulation of cytosolic calcium ion concentration / chemical synaptic transmission / perikaryon / calmodulin binding / postsynaptic density / cilium / synapse / dendrite / extracellular exosome / nucleoplasm / metal ion binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||||||||||||||||||||||||||
 Authors Authors | Gao, S. / Yao, X. / Yan, N. | |||||||||||||||||||||||||||||||||
| Funding support |  China, 1items China, 1items
| |||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Structural basis for human Ca1.2 inhibition by multiple drugs and the neurotoxin calciseptine. Authors: Shuai Gao / Xia Yao / Jiaofeng Chen / Gaoxingyu Huang / Xiao Fan / Lingfeng Xue / Zhangqiang Li / Tong Wu / Yupeng Zheng / Jian Huang / Xueqin Jin / Yan Wang / Zhifei Wang / Yong Yu / Lei ...Authors: Shuai Gao / Xia Yao / Jiaofeng Chen / Gaoxingyu Huang / Xiao Fan / Lingfeng Xue / Zhangqiang Li / Tong Wu / Yupeng Zheng / Jian Huang / Xueqin Jin / Yan Wang / Zhifei Wang / Yong Yu / Lei Liu / Xiaojing Pan / Chen Song / Nieng Yan /   Abstract: Ca1.2 channels play crucial roles in various neuronal and physiological processes. Here, we present cryo-EM structures of human Ca1.2, both in its apo form and in complex with several drugs, as well ...Ca1.2 channels play crucial roles in various neuronal and physiological processes. Here, we present cryo-EM structures of human Ca1.2, both in its apo form and in complex with several drugs, as well as the peptide neurotoxin calciseptine. Most structures, apo or bound to calciseptine, amlodipine, or a combination of amiodarone and sofosbuvir, exhibit a consistent inactivated conformation with a sealed gate, three up voltage-sensing domains (VSDs), and a down VSD. Calciseptine sits on the shoulder of the pore domain, away from the permeation path. In contrast, when pinaverium bromide, an antispasmodic drug, is inserted into a cavity reminiscent of the IFM-binding site in Na channels, a series of structural changes occur, including upward movement of VSD coupled with dilation of the selectivity filter and its surrounding segments in repeat III. Meanwhile, S4-5 merges with S5 to become a single helix, resulting in a widened but still non-conductive intracellular gate. | |||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8fhs.cif.gz 8fhs.cif.gz | 555.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8fhs.ent.gz pdb8fhs.ent.gz | 430.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8fhs.json.gz 8fhs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8fhs_validation.pdf.gz 8fhs_validation.pdf.gz | 2.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8fhs_full_validation.pdf.gz 8fhs_full_validation.pdf.gz | 2.2 MB | Display | |
| Data in XML |  8fhs_validation.xml.gz 8fhs_validation.xml.gz | 79.4 KB | Display | |
| Data in CIF |  8fhs_validation.cif.gz 8fhs_validation.cif.gz | 117.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fh/8fhs https://data.pdbj.org/pub/pdb/validation_reports/fh/8fhs ftp://data.pdbj.org/pub/pdb/validation_reports/fh/8fhs ftp://data.pdbj.org/pub/pdb/validation_reports/fh/8fhs | HTTPS FTP |
-Related structure data
| Related structure data |  29102MC  8we6C  8we7C  8we8C  8we9C  8weaC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Voltage-dependent L-type calcium channel subunit ... , 2 types, 2 molecules AC
| #1: Protein | Mass: 249242.219 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CACNA1C, CACH2, CACN2, CACNL1A1, CCHL1A1 / Production host: Homo sapiens (human) / Gene: CACNA1C, CACH2, CACN2, CACNL1A1, CCHL1A1 / Production host:  Homo sapiens (human) / References: UniProt: Q13936 Homo sapiens (human) / References: UniProt: Q13936 |
|---|---|
| #3: Protein | Mass: 54607.852 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CACNB3, CACNLB3 / Production host: Homo sapiens (human) / Gene: CACNB3, CACNLB3 / Production host:  Homo sapiens (human) / References: UniProt: P54284 Homo sapiens (human) / References: UniProt: P54284 |
-Protein , 1 types, 1 molecules D
| #2: Protein | Mass: 124692.469 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CACNA2D1, CACNL2A, CCHL2A, MHS3 / Production host: Homo sapiens (human) / Gene: CACNA2D1, CACNL2A, CCHL2A, MHS3 / Production host:  Homo sapiens (human) / References: UniProt: P54289 Homo sapiens (human) / References: UniProt: P54289 |
|---|
-Sugars , 4 types, 7 molecules 
| #4: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source | ||||
|---|---|---|---|---|---|
| #5: Polysaccharide | Source method: isolated from a genetically manipulated source #6: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #12: Sugar | |
-Non-polymers , 5 types, 10 molecules 
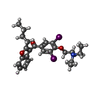
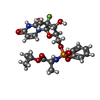






| #7: Chemical | | #8: Chemical | ChemComp-BBI / ( | #9: Chemical | ChemComp-WG6 / | #10: Chemical | #11: Chemical | ChemComp-CLR / |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cav1.2 / Type: COMPLEX / Entity ID: #1-#3 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 281 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2100 nm / Nominal defocus min: 1900 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20.1_4487: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 88794 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj


























