+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6rku | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli DNA Gyrase - DNA binding and cleavage domain in State 1 | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | ISOMERASE / Complex / DNA Gyrase / Inhibitor / DNA BINDING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of DNA-templated DNA replication / DNA topoisomerase type II (double strand cut, ATP-hydrolyzing) complex / DNA negative supercoiling activity / DNA topoisomerase type II (double strand cut, ATP-hydrolyzing) activity / DNA topoisomerase (ATP-hydrolysing) / DNA topological change / ATP-dependent activity, acting on DNA / DNA-templated DNA replication / chromosome / response to xenobiotic stimulus ...negative regulation of DNA-templated DNA replication / DNA topoisomerase type II (double strand cut, ATP-hydrolyzing) complex / DNA negative supercoiling activity / DNA topoisomerase type II (double strand cut, ATP-hydrolyzing) activity / DNA topoisomerase (ATP-hydrolysing) / DNA topological change / ATP-dependent activity, acting on DNA / DNA-templated DNA replication / chromosome / response to xenobiotic stimulus / response to antibiotic / DNA-templated transcription / DNA binding / ATP binding / identical protein binding / membrane / metal ion binding / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4 Å | ||||||||||||
 Authors Authors | Vanden Broeck, A. / Lamour, V. | ||||||||||||
| Funding support |  France, 3items France, 3items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Authors: Arnaud Vanden Broeck / Christophe Lotz / Julio Ortiz / Valérie Lamour /  Abstract: DNA gyrase is an essential enzyme involved in the homeostatic control of DNA supercoiling and the target of successful antibacterial compounds. Despite extensive studies, a detailed architecture of ...DNA gyrase is an essential enzyme involved in the homeostatic control of DNA supercoiling and the target of successful antibacterial compounds. Despite extensive studies, a detailed architecture of the full-length DNA gyrase from the model organism E. coli is still missing. Herein, we report the complete structure of the E. coli DNA gyrase nucleoprotein complex trapped by the antibiotic gepotidacin, using phase-plate single-particle cryo-electron microscopy. Our data unveil the structural and spatial organization of the functional domains, their connections and the position of the conserved GyrA-box motif. The deconvolution of two states of the DNA-binding/cleavage domain provides a better understanding of the allosteric movements of the enzyme complex. The local atomic resolution in the DNA-bound area reaching up to 3.0 Å enables the identification of the antibiotic density. Altogether, this study paves the way for the cryo-EM determination of gyrase complexes with antibiotics and opens perspectives for targeting conformational intermediates. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6rku.cif.gz 6rku.cif.gz | 379.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6rku.ent.gz pdb6rku.ent.gz | 287.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6rku.json.gz 6rku.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6rku_validation.pdf.gz 6rku_validation.pdf.gz | 888.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6rku_full_validation.pdf.gz 6rku_full_validation.pdf.gz | 902.8 KB | Display | |
| Data in XML |  6rku_validation.xml.gz 6rku_validation.xml.gz | 57.9 KB | Display | |
| Data in CIF |  6rku_validation.cif.gz 6rku_validation.cif.gz | 87.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rk/6rku https://data.pdbj.org/pub/pdb/validation_reports/rk/6rku ftp://data.pdbj.org/pub/pdb/validation_reports/rk/6rku ftp://data.pdbj.org/pub/pdb/validation_reports/rk/6rku | HTTPS FTP |
-Related structure data
| Related structure data |  4910MC  4909C  4912C  4913C  4914C  4915C  6rksC  6rkvC  6rkwC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
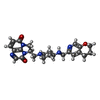
| #1: Protein | Mass: 97088.586 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: GyrA subunit DNA Binding and cleavage domain Source: (gene. exp.)  Gene: gyrA, hisW, nalA, parD, b2231, JW2225 / Production host:  #2: Protein | Mass: 90073.922 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: GyrB subunit DNA Binding and cleavage domain Source: (gene. exp.)  Gene: gyrB, acrB, cou, himB, hisU, nalC, parA, pcbA, b3699, JW5625 Production host:  #3: DNA chain | Mass: 4288.818 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)  #4: DNA chain | Mass: 5506.578 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)  #5: Chemical | ChemComp-JHN / ( | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Units: MEGADALTONS / Experimental value: NO | ||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 130000 X / Nominal defocus max: 800 nm / Nominal defocus min: 300 nm / Cs: 0.01 mm |
| Image recording | Electron dose: 50 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
| EM imaging optics | Energyfilter slit width: 20 eV / Phase plate: VOLTA PHASE PLATE |
| Image scans | Movie frames/image: 40 / Used frames/image: 3-40 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.12_2829: / Classification: refinement | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1572962 | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | ||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 60548 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | Space: REAL | ||||||||||||||||||||||||||||||||
| Atomic model building | 3D fitting-ID: 1 / Accession code: 3NUH / Initial refinement model-ID: 1 / PDB-ID: 3NUH / Source name: PDB / Type: experimental model
| ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj