+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Portal-tail of Vibrio cholerae typing phage mature VP1 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | phage / virus / vibrio cholera phage / VIRAL PROTEIN | ||||||||||||
| Biological species |   | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||
 Authors Authors | Liu HR / Pang H | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Three-dimensional structures of Vibrio cholerae typing podophage VP1 in two states. Authors: Hao Pang / Fenxia Fan / Jing Zheng / Hao Xiao / Zhixue Tan / Jingdong Song / Biao Kan / Hongrong Liu /  Abstract: Lytic podophages (VP1-VP5) play crucial roles in subtyping Vibrio cholerae O1 biotype El Tor. However, until now no structures of these phages have been available, which hindered our understanding of ...Lytic podophages (VP1-VP5) play crucial roles in subtyping Vibrio cholerae O1 biotype El Tor. However, until now no structures of these phages have been available, which hindered our understanding of the molecular mechanisms of infection and DNA release. Here, we determined the cryoelectron microscopy (cryo-EM) structures of mature and DNA-ejected VP1 structures at near-atomic and subnanometer resolutions, respectively. The VP1 head is composed of 415 copies of the major capsid protein gp7 and 11 turret-shaped spikes. The VP1 tail consists of an adapter, a nozzle, a slender ring, and a tail needle, and is flanked by three extended fibers I and six trimeric fibers II. Conformational changes of fiber II in DNA-ejected VP1 may cause the release of the tail needle and core proteins, forming an elongated tail channel. Our structures provide insights into the molecular mechanisms of infection and DNA release for podophages with a tail needle. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_60197.map.gz emd_60197.map.gz | 225.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-60197-v30.xml emd-60197-v30.xml emd-60197.xml emd-60197.xml | 17.7 KB 17.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_60197.png emd_60197.png | 71.8 KB | ||
| Filedesc metadata |  emd-60197.cif.gz emd-60197.cif.gz | 6.1 KB | ||
| Others |  emd_60197_half_map_1.map.gz emd_60197_half_map_1.map.gz emd_60197_half_map_2.map.gz emd_60197_half_map_2.map.gz | 226.2 MB 226.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-60197 http://ftp.pdbj.org/pub/emdb/structures/EMD-60197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60197 | HTTPS FTP |
-Related structure data
| Related structure data |  8zkkMC  8zkmC  9in6C M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_60197.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_60197.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
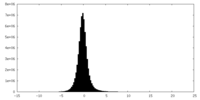
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_60197_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
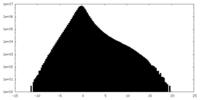
| Density Histograms |
-Half map: #1
| File | emd_60197_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
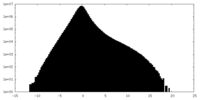
| Density Histograms |
- Sample components
Sample components
-Entire : Vibrio cholerae phage
| Entire | Name:  |
|---|---|
| Components |
|
-Supramolecule #1: Vibrio cholerae phage
| Supramolecule | Name: Vibrio cholerae phage / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 666 / Sci species name: Vibrio cholerae phage / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: nozzle gp16
| Macromolecule | Name: nozzle gp16 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 59.015762 KDa |
| Sequence | String: MEVTHKQFDL SGFLPDQAPD TLKSGAISRG FNVKPTILGW EKSGGFRETN TAPTNEKDFI FFWSPAIGDN RWFSGGDKTV QQVEGNVVS DVSRTGGYTA GSGRRWNAVN FNGVLLMNNE LDSPQYLAAS GKLEDFPNLP SNVRFRTVAV YKNFILGLGV N FGSGFLDD ...String: MEVTHKQFDL SGFLPDQAPD TLKSGAISRG FNVKPTILGW EKSGGFRETN TAPTNEKDFI FFWSPAIGDN RWFSGGDKTV QQVEGNVVS DVSRTGGYTA GSGRRWNAVN FNGVLLMNNE LDSPQYLAAS GKLEDFPNLP SNVRFRTVAV YKNFILGLGV N FGSGFLDD EIYWSHQADP GTMPPNWDYA NAASDSGRTP LPSEGYCVTS EELGSMNIVY KSDSIWTMQL IGGQWIFRFE NK FPGQGIL NKKSVVSFEG KHFVVTQKDI IVHDGYQVRS VADKRVRNFF FTDMNSDYFE RVFVVKDPRV AEVYVFYPSK NSV DGLCDR ALVWNWRDDV WSLLNLRPLK HAAYGYEITG VSITWDNFVG GWESTGLWQA DEDVAKYAPV LHYSFRDVPK LLAP TPQAL FIDEEIEAVW EREDIVIGSI SRDGVPYQDY ERNKSVSSIS FDVDTTEPFD VYIGYKGSLE DSVEWEFAGT VNPME DKRL FCLLTAGLFS MRIISKAQTF ILRSYKITYE FAGEMWS |
-Macromolecule #2: portal gp5
| Macromolecule | Name: portal gp5 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 73.263602 KDa |
| Sequence | String: MEILYTGASE SLHSTILKAL LERIDLGDTF ISDNYTRWQA TERYYMMYKI PNKKDKAAIE KWNKGDTDFK SLVMPYSYAQ LMTAHAYMV NVFLNRDPIF QTDSLNGDGT ERELALESML QYQVKAGEME PSLLVWFMDA LRYGVGVLGD YWEEHVFHQT V FEEEEEII ...String: MEILYTGASE SLHSTILKAL LERIDLGDTF ISDNYTRWQA TERYYMMYKI PNKKDKAAIE KWNKGDTDFK SLVMPYSYAQ LMTAHAYMV NVFLNRDPIF QTDSLNGDGT ERELALESML QYQVKAGEME PSLLVWFMDA LRYGVGVLGD YWEEHVFHQT V FEEEEEII DGVPTGNMKK VKKTRVVKGY EGCKTFNVMV YDFIPDPRVA LCKYQEGEFF GRRLDLNVLD LKKGAKFGKY FN VEHAEAL VAASKEEMYR RDPSIGQQRS LKDSTMTPKG KQVGDISCVE IFVRLVPKDW GLGDSEFPEM WVFTVADKKY IVA AEPVNT LDDKFPFHIL ECEIDGYMNK SRGLLEISAP MNDILTWLFD SHMYNKRQIM NNQFIGDPSA LVVKDVESKE PGKF IRLRP TAYGRDVRSI ISQLPVTDVT AQNIQDVQVV ERNMQRIVGV NDDVAGQSSP SSRRSATEFR GTTSFASSRL ANLAY FFSV TGFRSLAKSL IVKTQQLYTV EMKVKVAGDN IKGAQSIIVK PEDISGQFDI MPVDGTLPVD RMAQAQFWMQ IMSMVA GNP VLGAEYRLGD IFSYTARLAG LKGIDKMKIR ILDDDQILAL ILAQQKGGAD VQPTGQPTTQ GVGNPTGVNE PAPSVTQ GT PGLSGLQALM |
-Macromolecule #3: adaptor gp12
| Macromolecule | Name: adaptor gp12 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.59285 KDa |
| Sequence | String: MDKATLVKTI AYRMGNVKGQ DTAIDFELAL SIERLEGQEF VPWFLLSENN FFEGTAQENR IPVPRGFIRE YEEGSLYLRR VAGTGKCLI KKSQDQLLKY EGMTGEPSHY SLTNQYFRIY PVPQEDFKVE LLFYRKSSTL NVEDNPWYEY AAELLVAETI W AMLSARRD ...String: MDKATLVKTI AYRMGNVKGQ DTAIDFELAL SIERLEGQEF VPWFLLSENN FFEGTAQENR IPVPRGFIRE YEEGSLYLRR VAGTGKCLI KKSQDQLLKY EGMTGEPSHY SLTNQYFRIY PVPQEDFKVE LLFYRKSSTL NVEDNPWYEY AAELLVAETI W AMLSARRD KMADYWKSVA ADQMRRLTIL DAERRLANQE IFMG |
-Macromolecule #4: ring protein gp10
| Macromolecule | Name: ring protein gp10 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 26.311119 KDa |
| Sequence | String: MSSWDRFDTP WDSIIDESTW EHYTFKYDAS FEAFSSMEVD EDLTITVSVL FASTSDNTVT VGQVLGLTMD NRAGSYFGAG SSWTSEAAV NNEAGSGFQS SHALQLSYSV EDGVISSFGS EAFCSFYNTV SFESSSDVQA AVNSLYNLDV LFSSGSGDSE Q HYVVFGET ...String: MSSWDRFDTP WDSIIDESTW EHYTFKYDAS FEAFSSMEVD EDLTITVSVL FASTSDNTVT VGQVLGLTMD NRAGSYFGAG SSWTSEAAV NNEAGSGFQS SHALQLSYSV EDGVISSFGS EAFCSFYNTV SFESSSDVQA AVNSLYNLDV LFSSGSGDSE Q HYVVFGET ASFESLAEHE TSSQYITHVE CMFNSVVEFE VKTYDWGRPV KPVGSDWSTD TPVVGVWVNE IYNGNKDWGE S |
-Macromolecule #5: gp13
| Macromolecule | Name: gp13 / type: protein_or_peptide / ID: 5 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10.025203 KDa |
| Sequence | String: MALETWDANS TPATLNTAWP EATDPLNKGD DHIRLLKTVV VNFWNKVFDG SKLKTAVVPA AVNSATAGSG FGGFRYQVVN NSDGTKTLR LFTS |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 32.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 47456 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)