[English] 日本語
 Yorodumi
Yorodumi- EMDB-4876: Cryo-EM structure of the anti-feeding prophage (AFP) baseplate in... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4876 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the anti-feeding prophage (AFP) baseplate in contracted state | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Anti-feeding prophage / secretion system / AFP / contractile / VIRUS LIKE PARTICLE / baseplate / contracted | |||||||||
| Function / homology | : / Tail sheath protein, subtilisin-like domain / Phage tail sheath protein subtilisin-like domain / Tail sheath protein, C-terminal domain / Phage tail sheath C-terminal domain / Afp4 / Afp3 / Afp2 Function and homology information Function and homology information | |||||||||
| Biological species |  Serratia entomophila (bacteria) Serratia entomophila (bacteria) | |||||||||
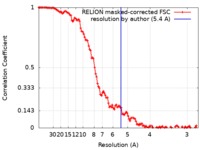
| Method | single particle reconstruction / cryo EM / Resolution: 5.4 Å | |||||||||
 Authors Authors | Desfosses A | |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2019 Journal: Nat Microbiol / Year: 2019Title: Atomic structures of an entire contractile injection system in both the extended and contracted states. Authors: Ambroise Desfosses / Hariprasad Venugopal / Tapan Joshi / Jan Felix / Matthew Jessop / Hyengseop Jeong / Jaekyung Hyun / J Bernard Heymann / Mark R H Hurst / Irina Gutsche / Alok K Mitra /       Abstract: Contractile injection systems are sophisticated multiprotein nanomachines that puncture target cell membranes. Although the number of atomic-resolution insights into contractile bacteriophage tails, ...Contractile injection systems are sophisticated multiprotein nanomachines that puncture target cell membranes. Although the number of atomic-resolution insights into contractile bacteriophage tails, bacterial type six secretion systems and R-pyocins is rapidly increasing, structural information on the contraction of bacterial phage-like protein-translocation structures directed towards eukaryotic hosts is scarce. Here, we characterize the antifeeding prophage AFP from Serratia entomophila by cryo-electron microscopy. We present the high-resolution structure of the entire AFP particle in the extended state, trace 11 protein chains de novo from the apical cap to the needle tip, describe localization variants and perform specific structural comparisons with related systems. We analyse inter-subunit interactions and highlight their universal conservation within contractile injection systems while revealing the specificities of AFP. Furthermore, we provide the structure of the AFP sheath-baseplate complex in a contracted state. This study reveals atomic details of interaction networks that accompany and define the contraction mechanism of toxin-delivery tailocins, offering a comprehensive framework for understanding their mode of action and for their possible adaptation as biocontrol agents. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4876.map.gz emd_4876.map.gz | 74 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4876-v30.xml emd-4876-v30.xml emd-4876.xml emd-4876.xml | 14.5 KB 14.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4876_fsc.xml emd_4876_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_4876.png emd_4876.png | 96.6 KB | ||
| Filedesc metadata |  emd-4876.cif.gz emd-4876.cif.gz | 6.1 KB | ||
| Others |  emd_4876_half_map_1.map.gz emd_4876_half_map_1.map.gz emd_4876_half_map_2.map.gz emd_4876_half_map_2.map.gz | 100.5 MB 100.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4876 http://ftp.pdbj.org/pub/emdb/structures/EMD-4876 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4876 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4876 | HTTPS FTP |
-Related structure data
| Related structure data |  6rglMC  4782C  4783C  4784C  4800C  4801C  4802C  4803C  4859C  4871C  6raoC  6rapC  6rbkC  6rbnC  6rc8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_4876.map.gz / Format: CCP4 / Size: 129.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4876.map.gz / Format: CCP4 / Size: 129.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.397 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Supplemental map: emd 4876 half map 1.map
| File | emd_4876_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Supplemental map: emd 4876 half map 2.map
| File | emd_4876_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of the anti-feeding prophage (AFP) baseplate in...
| Entire | Name: Cryo-EM structure of the anti-feeding prophage (AFP) baseplate in contracted state |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the anti-feeding prophage (AFP) baseplate in...
| Supramolecule | Name: Cryo-EM structure of the anti-feeding prophage (AFP) baseplate in contracted state type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Serratia entomophila (bacteria) Serratia entomophila (bacteria) |
-Macromolecule #1: Afp2
| Macromolecule | Name: Afp2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Serratia entomophila (bacteria) Serratia entomophila (bacteria) |
| Molecular weight | Theoretical: 38.784355 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTVTTTYPGV YLSEDAVSSF SVNSAATAVP LFAYDSENTN TINKPIQVFR NWAEFTVEYP TPLEDAFYTS LSLWFMHGGG KCYLVNEAN IADAVAQYDD ITLIVAAGTD TTTYTAFTTV VGQGYRIFGL FDGPKEKIAG TAKPDEVMEE YPTSPFGAVF Y PWGTLASG ...String: MTVTTTYPGV YLSEDAVSSF SVNSAATAVP LFAYDSENTN TINKPIQVFR NWAEFTVEYP TPLEDAFYTS LSLWFMHGGG KCYLVNEAN IADAVAQYDD ITLIVAAGTD TTTYTAFTTV VGQGYRIFGL FDGPKEKIAG TAKPDEVMEE YPTSPFGAVF Y PWGTLASG AAVPPSAIAA ASITQTDRTR GVWKAPANQA VNGVTPAFAV SDDFQGKYNQ GKALNMIRTF SGQGTVVWGA RT LEDSDNW RYIPVRRLFN AVERDIQKSL NKLVFEPNSQ PTWQRVKAAV DSYLHSLWQQ GALAGNTPAD AWFVQVGKDL TMT QEEINQ GKMIIKIGLA AVRPAEFIIL QFSQDIAQ UniProtKB: Afp2 |
-Macromolecule #2: Afp3
| Macromolecule | Name: Afp3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Serratia entomophila (bacteria) Serratia entomophila (bacteria) |
| Molecular weight | Theoretical: 48.777566 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MATVTSVPGV YIEEDASPAM SVSASATAVP LFVARFTPLK PELAGVITRI GSWLDYTILF DSNVPSSARV TVSSTAVEPS PEFDALETA SSKATTTYTY QIDDTEVVDP TASVALRLYF QNGGGPCYLY PLEKADDNGP LAALPDLIDE VGEITLLASP D PDETYRTA ...String: MATVTSVPGV YIEEDASPAM SVSASATAVP LFVARFTPLK PELAGVITRI GSWLDYTILF DSNVPSSARV TVSSTAVEPS PEFDALETA SSKATTTYTY QIDDTEVVDP TASVALRLYF QNGGGPCYLY PLEKADDNGP LAALPDLIDE VGEITLLASP D PDETYRTA VYGALAASLD QHKGYFLLAD SVNGDAPSAV GGSAQVAVYY PNVEVPHTRK LDDAEVAIDG YLDDEGKAVT TL AALRVVN TEFAGEIAQS LSGDLSAPLS LPPSALIAGV YGKTDGERGV WKAPANVVLN GVSDVSVRVT NEQQAELNPK GIN VIRHFS DRGLVVWGSR TQKDDDDWRY IPVRRLFDAA ERDIKKALQP MVFEPNSQLT WKRVQTAIDN YLYRLWQQGA LAGN KAEEA YFVRVGKGIT MTQDEINQGK MIIQVGMAAV RPAEFIILKF TQDMSQ UniProtKB: Afp3 |
-Macromolecule #3: Afp4
| Macromolecule | Name: Afp4 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Serratia entomophila (bacteria) Serratia entomophila (bacteria) |
| Molecular weight | Theoretical: 45.565633 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTMVLPGVSY NETLLTQASN DDPVTMPLFI GYTPPDTAIP VTVMQPVSVG SLTQANSLFG QRGTLAYSLR HFFENGGLQC YVLPLGPGK GEPAARLQEL IAALQTPQML ETLLADDKTG LVLVPELSEL NEVSSTSLSA EGVDAAEVDA DALWYQGWQV L LTLCRQAP ...String: MTMVLPGVSY NETLLTQASN DDPVTMPLFI GYTPPDTAIP VTVMQPVSVG SLTQANSLFG QRGTLAYSLR HFFENGGLQC YVLPLGPGK GEPAARLQEL IAALQTPQML ETLLADDKTG LVLVPELSEL NEVSSTSLSA EGVDAAEVDA DALWYQGWQV L LTLCRQAP QRFALLELPE DPASAVTLTQ QSFSADQCQR GAAWWPRLET SYQDESSAPV VLSPLPAVAA AIQRSAHDNG VW KAPANIA LAKTRRPTQS ILTSQALLDN QGVSCNLIRS FVGKGVRLWG CRTLLNEENT AWRYIQIRLL VSSVEHYLSK LAR AYLFEP NTAPTWMKLK GQVWTWLRQQ WLAGAFFGTV EDEAFSLSIG LDETMTEDDI RHGKMILQVR LALLAPAEFI AISL TLDLR DGTASAQTGG QS UniProtKB: Afp4 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 27.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)