+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human Wnt3a bound to WLS and CALR | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | SIGNALING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationWnt signaling pathway involved in forebrain neuroblast division / positive regulation of dermatome development / calcium ion transmembrane transport via low voltage-gated calcium channel / positive regulation of collateral sprouting in absence of injury / positive regulation of mesodermal cell fate specification / paraxial mesodermal cell fate commitment / axis elongation involved in somitogenesis / cell proliferation in midbrain / spinal cord association neuron differentiation / Wnt protein secretion ...Wnt signaling pathway involved in forebrain neuroblast division / positive regulation of dermatome development / calcium ion transmembrane transport via low voltage-gated calcium channel / positive regulation of collateral sprouting in absence of injury / positive regulation of mesodermal cell fate specification / paraxial mesodermal cell fate commitment / axis elongation involved in somitogenesis / cell proliferation in midbrain / spinal cord association neuron differentiation / Wnt protein secretion / Formation of the posterior neural plate / Calnexin/calreticulin cycle / COP9 signalosome assembly / Wnt-Frizzled-LRP5/6 complex / cytolytic granule / positive regulation of cell-cell adhesion mediated by cadherin / positive regulation of Wnt protein secretion / Negative regulation of TCF-dependent signaling by WNT ligand antagonists / positive regulation of dendritic cell chemotaxis / synaptic vesicle recycling / WNT ligand biogenesis and trafficking / Signaling by RNF43 mutants / Assembly of Viral Components at the Budding Site / positive regulation of cardiac muscle cell differentiation / ATF6 (ATF6-alpha) activates chaperone genes / negative regulation of trophoblast cell migration / cortical granule / cell proliferation in forebrain / negative regulation of axon extension involved in axon guidance / nuclear receptor-mediated glucocorticoid signaling pathway / cellular response to electrical stimulus / complement component C1q complex binding / regulation of meiotic nuclear division / negative regulation of retinoic acid receptor signaling pathway / secondary palate development / response to glycoside / Specification of the neural plate border / endoplasmic reticulum quality control compartment / cementum mineralization / sequestering of calcium ion / somatic stem cell division / cardiac muscle cell fate commitment / sarcoplasmic reticulum lumen / protein folding in endoplasmic reticulum / co-receptor binding / hormone binding / presynapse assembly / non-canonical Wnt signaling pathway / hindbrain development / positive regulation of skeletal muscle tissue development / negative regulation of intracellular steroid hormone receptor signaling pathway / negative regulation of dopaminergic neuron differentiation / Wnt-protein binding / nuclear export signal receptor activity / midbrain dopaminergic neuron differentiation / mammary gland development / dorsal/ventral neural tube patterning / regulation of postsynapse to nucleus signaling pathway / cardiac muscle cell differentiation / exocrine pancreas development / post-anal tail morphogenesis / molecular sequestering activity / positive regulation of neural precursor cell proliferation / frizzled binding / Class B/2 (Secretin family receptors) / positive regulation of hepatocyte proliferation / myoblast differentiation / Disassembly of the destruction complex and recruitment of AXIN to the membrane / anterior/posterior axis specification / Scavenging by Class A Receptors / inner ear morphogenesis / Scavenging by Class F Receptors / protein maturation by protein folding / midbrain development / cortical actin cytoskeleton organization / nuclear androgen receptor binding / cellular response to lithium ion / regulation of synapse organization / negative regulation of fat cell differentiation / organelle membrane / fat cell differentiation / B cell proliferation / heart looping / Formation of paraxial mesoderm / positive regulation of receptor internalization / skeletal muscle cell differentiation / response to testosterone / hemopoiesis / positive regulation of Wnt signaling pathway / mesoderm formation / protein localization to nucleus / regulation of presynapse assembly / negative regulation of neuron differentiation / cell fate commitment / endomembrane system / positive regulation of cell cycle / smooth endoplasmic reticulum / canonical Wnt signaling pathway / Transcriptional and post-translational regulation of MITF-M expression and activity / regulation of microtubule cytoskeleton organization Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||
 Authors Authors | Qi X / Hu Q / Li X | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Molecular basis of Wnt biogenesis, secretion, and Wnt7-specific signaling. Authors: Xiaofeng Qi / Qinli Hu / Nadia Elghobashi-Meinhardt / Tao Long / Hongwen Chen / Xiaochun Li /   Abstract: Wnt proteins are enzymatically lipidated by Porcupine (PORCN) in the ER and bind to Wntless (WLS) for intracellular transport and secretion. Mechanisms governing the transfer of these low-solubility ...Wnt proteins are enzymatically lipidated by Porcupine (PORCN) in the ER and bind to Wntless (WLS) for intracellular transport and secretion. Mechanisms governing the transfer of these low-solubility Wnts from the ER to the extracellular space remain unclear. Through structural and functional analyses of Wnt7a, a crucial Wnt involved in central nervous system angiogenesis and blood-brain barrier maintenance, we have elucidated the principles of Wnt biogenesis and Wnt7-specific signaling. The Wnt7a-WLS complex binds to calreticulin (CALR), revealing that CALR functions as a chaperone to facilitate Wnt transfer from PORCN to WLS during Wnt biogenesis. Our structures, functional analyses, and molecular dynamics simulations demonstrate that a phospholipid in the core of Wnt-bound WLS regulates the association and dissociation between Wnt and WLS, suggesting a lipid-mediated Wnt secretion mechanism. Finally, the structure of Wnt7a bound to RECK, a cell-surface Wnt7 co-receptor, reveals how RECK engages the N-terminal domain of Wnt7a to activate Wnt7-specific signaling. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41767.map.gz emd_41767.map.gz | 78.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41767-v30.xml emd-41767-v30.xml emd-41767.xml emd-41767.xml | 16.8 KB 16.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41767.png emd_41767.png | 55.2 KB | ||
| Filedesc metadata |  emd-41767.cif.gz emd-41767.cif.gz | 6.4 KB | ||
| Others |  emd_41767_half_map_1.map.gz emd_41767_half_map_1.map.gz emd_41767_half_map_2.map.gz emd_41767_half_map_2.map.gz | 77.6 MB 77.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41767 http://ftp.pdbj.org/pub/emdb/structures/EMD-41767 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41767 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41767 | HTTPS FTP |
-Validation report
| Summary document |  emd_41767_validation.pdf.gz emd_41767_validation.pdf.gz | 940.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41767_full_validation.pdf.gz emd_41767_full_validation.pdf.gz | 940.4 KB | Display | |
| Data in XML |  emd_41767_validation.xml.gz emd_41767_validation.xml.gz | 12.9 KB | Display | |
| Data in CIF |  emd_41767_validation.cif.gz emd_41767_validation.cif.gz | 15.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41767 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41767 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41767 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41767 | HTTPS FTP |
-Related structure data
| Related structure data |  8tzrM  8tzoC  8tzpC  8tzsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41767.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41767.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_41767_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
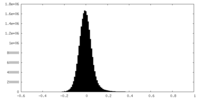
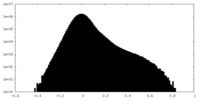
| Density Histograms |
-Half map: #1
| File | emd_41767_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
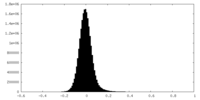
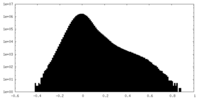
| Density Histograms |
- Sample components
Sample components
-Entire : Wnt3a-WLS-CALR Complex
| Entire | Name: Wnt3a-WLS-CALR Complex |
|---|---|
| Components |
|
-Supramolecule #1: Wnt3a-WLS-CALR Complex
| Supramolecule | Name: Wnt3a-WLS-CALR Complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Protein Wnt-3a
| Macromolecule | Name: Protein Wnt-3a / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.421832 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAPLGYFLLL CSLKQALGSY PIWWSLAVGP QYSSLGSQPI LCASIPGLVP KQLRFCRNYV EIMPSVAEGI KIGIQECQHQ FRGRRWNCT TVHDSLAIFG PVLDKATRES AFVHAIASAG VAFAVTRSCA EGTAAICGCS SRHQGSPGKG WKWGGCSEDI E FGGMVSRE ...String: MAPLGYFLLL CSLKQALGSY PIWWSLAVGP QYSSLGSQPI LCASIPGLVP KQLRFCRNYV EIMPSVAEGI KIGIQECQHQ FRGRRWNCT TVHDSLAIFG PVLDKATRES AFVHAIASAG VAFAVTRSCA EGTAAICGCS SRHQGSPGKG WKWGGCSEDI E FGGMVSRE FADARENRPD ARSAMNRHNN EAGRQAIASH MHLKCKCHGL SGSCEVKTCW WSQPDFRAIG DFLKDKYDSA SE MVVEKHR ESRGWVETLR PRYTYFKVPT ERDLVYYEAS PNFCEPNPET GSFGTRDRTC NVSSHGIDGC DLLCCGRGHN ARA ERRREK CRCVFHWCCY VSCQECTRVY DVHTCK UniProtKB: Protein Wnt-3a |
-Macromolecule #2: Protein wntless homolog
| Macromolecule | Name: Protein wntless homolog / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 62.317973 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAGAIIENMS TKKLCIVGGI LLVFQIIAFL VGGLIAPGPT TAVSYMSVKC VDARKNHHKT KWFVPWGPNH CDKIRDIEEA IPREIEAND IVFSVHIPLP HMEMSPWFQF MLFILQLDIA FKLNNQIREN AEVSMDVSLA YRDDAFAEWT EMAHERVPRK L KCTFTSPK ...String: MAGAIIENMS TKKLCIVGGI LLVFQIIAFL VGGLIAPGPT TAVSYMSVKC VDARKNHHKT KWFVPWGPNH CDKIRDIEEA IPREIEAND IVFSVHIPLP HMEMSPWFQF MLFILQLDIA FKLNNQIREN AEVSMDVSLA YRDDAFAEWT EMAHERVPRK L KCTFTSPK TPEHEGRYYE CDVLPFMEIG SVAHKFYLLN IRLPVNEKKK INVGIGEIKD IRLVGIHQNG GFTKVWFAMK TF LTPSIFI IMVWYWRRIT MMSRPPVLLE KVIFALGISM TFINIPVEWF SIGFDWTWML LFGDIRQGIF YAMLLSFWII FCG EHMMDQ HERNHIAGYW KQVGPIAVGS FCLFIFDMCE RGVQLTNPFY SIWTTDIGTE LAMAFIIVAG ICLCLYFLFL CFMV FQVFR NISGKQSSLP AMSKVRRLHY EGLIFRFKFL MLITLACAAM TVIFFIVSQV TEGHWKWGGV TVQVNSAFFT GIYGM WNLY VFALMFLYAP SHKNYGEDQS NGDLGVHSGE ELQLTTTITH VDGPTEIYKL TRKEAQE UniProtKB: Protein wntless homolog |
-Macromolecule #3: Calreticulin
| Macromolecule | Name: Calreticulin / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 48.198379 KDa |
| Sequence | String: MLLSVPLLLG LLGLAVAEPA VYFKEQFLDG DGWTSRWIES KHKSDFGKFV LSSGKFYGDE EKDKGLQTSQ DARFYALSAS FEPFSNKGQ TLVVQFTVKH EQNIDCGGGY VKLFPNSLDQ TDMHGDSEYN IMFGPDICGP GTKKVHVIFN YKGKNVLINK D IRCKDDEF ...String: MLLSVPLLLG LLGLAVAEPA VYFKEQFLDG DGWTSRWIES KHKSDFGKFV LSSGKFYGDE EKDKGLQTSQ DARFYALSAS FEPFSNKGQ TLVVQFTVKH EQNIDCGGGY VKLFPNSLDQ TDMHGDSEYN IMFGPDICGP GTKKVHVIFN YKGKNVLINK D IRCKDDEF THLYTLIVRP DNTYEVKIDN SQVESGSLED DWDFLPPKKI KDPDASKPED WDERAKIDDP TDSKPEDWDK PE HIPDPDA KKPEDWDEEM DGEWEPPVIQ NPEYKGEWKP RQIDNPDYKG TWIHPEIDNP EYSPDPSIYA YDNFGVLGLD LWQ VKSGTI FDNFLITNDE AYAEEFGNET WGVTKAAEKQ MKDKQDEEQR LKEEEEDKKR KEEEEAEDKE DDEDKDEDEE DEED KEEDE EEDVPGQAKD EL UniProtKB: Calreticulin |
-Macromolecule #5: PALMITOLEIC ACID
| Macromolecule | Name: PALMITOLEIC ACID / type: ligand / ID: 5 / Number of copies: 1 / Formula: PAM |
|---|---|
| Molecular weight | Theoretical: 254.408 Da |
| Chemical component information | 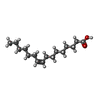 ChemComp-PAM: |
-Macromolecule #6: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 6 / Number of copies: 1 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 113802 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)