+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31475 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | eIF2B-SFSV NSs-2-eIF2 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | translation initiation factor / viral protein / TRANSLATION | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationtranslation initiation ternary complex / regulation of translation in response to endoplasmic reticulum stress / glial limiting end-foot / HRI-mediated signaling / eukaryotic translation initiation factor 2B complex / response to manganese-induced endoplasmic reticulum stress / Cellular response to mitochondrial stress / positive regulation of type B pancreatic cell apoptotic process / Response of EIF2AK1 (HRI) to heme deficiency / Recycling of eIF2:GDP ...translation initiation ternary complex / regulation of translation in response to endoplasmic reticulum stress / glial limiting end-foot / HRI-mediated signaling / eukaryotic translation initiation factor 2B complex / response to manganese-induced endoplasmic reticulum stress / Cellular response to mitochondrial stress / positive regulation of type B pancreatic cell apoptotic process / Response of EIF2AK1 (HRI) to heme deficiency / Recycling of eIF2:GDP / methionyl-initiator methionine tRNA binding / negative regulation of translational initiation in response to stress / PERK-mediated unfolded protein response / PERK regulates gene expression / response to kainic acid / eukaryotic translation initiation factor 2 complex / cytoplasmic translational initiation / regulation of translational initiation in response to stress / translation factor activity, RNA binding / formation of translation preinitiation complex / astrocyte development / guanyl-nucleotide exchange factor complex / eukaryotic 48S preinitiation complex / astrocyte differentiation / oligodendrocyte development / protein-synthesizing GTPase / regulation of translational initiation / Formation of the ternary complex, and subsequently, the 43S complex / Ribosomal scanning and start codon recognition / Translation initiation complex formation / positive regulation of translational initiation / Response of EIF2AK4 (GCN2) to amino acid deficiency / GTP hydrolysis and joining of the 60S ribosomal subunit / response to glucose / L13a-mediated translational silencing of Ceruloplasmin expression / ovarian follicle development / mitophagy / myelination / translation initiation factor binding / translation initiation factor activity / stress granule assembly / cellular response to amino acid starvation / guanyl-nucleotide exchange factor activity / response to endoplasmic reticulum stress / central nervous system development / hippocampus development / translational initiation / response to peptide hormone / PKR-mediated signaling / ABC-family proteins mediated transport / cytoplasmic stress granule / cellular response to UV / T cell receptor signaling pathway / regulation of translation / cellular response to heat / ribosome binding / response to heat / cellular response to oxidative stress / positive regulation of apoptotic process / cadherin binding / GTPase activity / synapse / GTP binding / mitochondrion / RNA binding / extracellular exosome / ATP binding / identical protein binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Sandfly fever sicilian virus Sandfly fever sicilian virus | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.59 Å | ||||||||||||
 Authors Authors | Kashiwagi K / Ito T | ||||||||||||
| Funding support |  Japan, 3 items Japan, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: eIF2B-capturing viral protein NSs suppresses the integrated stress response. Authors: Kazuhiro Kashiwagi / Yuichi Shichino / Tatsuya Osaki / Ayako Sakamoto / Madoka Nishimoto / Mari Takahashi / Mari Mito / Friedemann Weber / Yoshiho Ikeuchi / Shintaro Iwasaki / Takuhiro Ito /   Abstract: Various stressors such as viral infection lead to the suppression of cap-dependent translation and the activation of the integrated stress response (ISR), since the stress-induced phosphorylated ...Various stressors such as viral infection lead to the suppression of cap-dependent translation and the activation of the integrated stress response (ISR), since the stress-induced phosphorylated eukaryotic translation initiation factor 2 [eIF2(αP)] tightly binds to eIF2B to prevent it from exchanging guanine nucleotide molecules on its substrate, unphosphorylated eIF2. Sandfly fever Sicilian virus (SFSV) evades this cap-dependent translation suppression through the interaction between its nonstructural protein NSs and host eIF2B. However, its precise mechanism has remained unclear. Here, our cryo-electron microscopy (cryo-EM) analysis reveals that SFSV NSs binds to the α-subunit of eIF2B in a competitive manner with eIF2(αP). Together with SFSV NSs, eIF2B retains nucleotide exchange activity even in the presence of eIF2(αP), in line with the cryo-EM structures of the eIF2B•SFSV NSs•unphosphorylated eIF2 complex. A genome-wide ribosome profiling analysis clarified that SFSV NSs expressed in cultured human cells attenuates the ISR triggered by thapsigargin, an endoplasmic reticulum stress inducer. Furthermore, SFSV NSs introduced in rat hippocampal neurons and human induced-pluripotent stem (iPS) cell-derived motor neurons exhibits neuroprotective effects against the ISR-inducing stress. Since ISR inhibition is beneficial in various neurological disease models, SFSV NSs may be a promising therapeutic ISR inhibitor. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
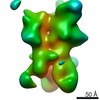
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31475.map.gz emd_31475.map.gz | 208.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31475-v30.xml emd-31475-v30.xml emd-31475.xml emd-31475.xml | 25.2 KB 25.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31475.png emd_31475.png | 94.5 KB | ||
| Filedesc metadata |  emd-31475.cif.gz emd-31475.cif.gz | 8.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31475 http://ftp.pdbj.org/pub/emdb/structures/EMD-31475 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31475 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31475 | HTTPS FTP |
-Related structure data
| Related structure data |  7f67MC  7f64C  7f66C  7vlkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31475.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31475.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.829 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
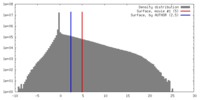
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Complex of eIF2B, eIF2, and SFSV NSs
+Supramolecule #1: Complex of eIF2B, eIF2, and SFSV NSs
+Supramolecule #2: eIF2B, eIF2
+Supramolecule #3: SFSV NSs
+Macromolecule #1: Translation initiation factor eIF-2B subunit alpha
+Macromolecule #2: Translation initiation factor eIF-2B subunit beta
+Macromolecule #3: Translation initiation factor eIF-2B subunit gamma
+Macromolecule #4: Translation initiation factor eIF-2B subunit delta
+Macromolecule #5: Translation initiation factor eIF-2B subunit epsilon
+Macromolecule #6: Non-structural protein NS-S
+Macromolecule #7: Eukaryotic translation initiation factor 2 subunit 1
+Macromolecule #8: eIF2beta
+Macromolecule #9: Eukaryotic translation initiation factor 2 subunit 3
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)