[English] 日本語
 Yorodumi
Yorodumi- EMDB-30972: Serotonin-bound Serotonin 1A (5-HT1A) receptor-Gi protein complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30972 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Serotonin-bound Serotonin 1A (5-HT1A) receptor-Gi protein complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Serotonin / 5-HT1A / GPCR / Complex / G protein / Gi / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of serotonin secretion / Gi/o-coupled serotonin receptor activity / regulation of hormone secretion / regulation of behavior / receptor-receptor interaction / Serotonin receptors / regulation of dopamine metabolic process / serotonin receptor activity / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway ...regulation of serotonin secretion / Gi/o-coupled serotonin receptor activity / regulation of hormone secretion / regulation of behavior / receptor-receptor interaction / Serotonin receptors / regulation of dopamine metabolic process / serotonin receptor activity / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway / neurotransmitter receptor activity / serotonin metabolic process / serotonin binding / gamma-aminobutyric acid signaling pathway / exploration behavior / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / regulation of vasoconstriction / behavioral fear response / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / electron transport chain / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / GDP binding / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / G protein activity / retina development in camera-type eye / GTPase binding / Ca2+ pathway / fibroblast proliferation / midbody / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / chemical synaptic transmission / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Ras protein signal transduction / electron transfer activity / periplasmic space / Extra-nuclear estrogen signaling / cell population proliferation / ciliary basal body / iron ion binding / G protein-coupled receptor signaling pathway Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Xu P / Huang S | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structural insights into the lipid and ligand regulation of serotonin receptors. Authors: Peiyu Xu / Sijie Huang / Huibing Zhang / Chunyou Mao / X Edward Zhou / Xi Cheng / Icaro A Simon / Dan-Dan Shen / Hsin-Yung Yen / Carol V Robinson / Kasper Harpsøe / Bo Svensson / Jia Guo / ...Authors: Peiyu Xu / Sijie Huang / Huibing Zhang / Chunyou Mao / X Edward Zhou / Xi Cheng / Icaro A Simon / Dan-Dan Shen / Hsin-Yung Yen / Carol V Robinson / Kasper Harpsøe / Bo Svensson / Jia Guo / Hualiang Jiang / David E Gloriam / Karsten Melcher / Yi Jiang / Yan Zhang / H Eric Xu /      Abstract: Serotonin, or 5-hydroxytryptamine (5-HT), is an important neurotransmitter that activates the largest subtype family of G-protein-coupled receptors. Drugs that target 5-HT, 5-HT, 5-HT and other 5-HT ...Serotonin, or 5-hydroxytryptamine (5-HT), is an important neurotransmitter that activates the largest subtype family of G-protein-coupled receptors. Drugs that target 5-HT, 5-HT, 5-HT and other 5-HT receptors are used to treat numerous disorders. 5-HT receptors have high levels of basal activity and are subject to regulation by lipids, but the structural basis for the lipid regulation and basal activation of these receptors and the pan-agonism of 5-HT remains unclear. Here we report five structures of 5-HT receptor-G-protein complexes: 5-HT in the apo state, bound to 5-HT or bound to the antipsychotic drug aripiprazole; 5-HT bound to 5-HT; and 5-HT in complex with a 5-HT- and 5-HT-selective agonist, BRL-54443. Notably, the phospholipid phosphatidylinositol 4-phosphate is present at the G-protein-5-HT interface, and is able to increase 5-HT-mediated G-protein activity. The receptor transmembrane domain is surrounded by cholesterol molecules-particularly in the case of 5-HT, in which cholesterol molecules are directly involved in shaping the ligand-binding pocket that determines the specificity for aripiprazol. Within the ligand-binding pocket of apo-5-HT are structured water molecules that mimic 5-HT to activate the receptor. Together, our results address a long-standing question of how lipids and water molecules regulate G-protein-coupled receptors, reveal how 5-HT acts as a pan-agonist, and identify the determinants of drug recognition in 5-HT receptors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30972.map.gz emd_30972.map.gz | 25.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30972-v30.xml emd-30972-v30.xml emd-30972.xml emd-30972.xml | 16.3 KB 16.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30972.png emd_30972.png | 55.9 KB | ||
| Filedesc metadata |  emd-30972.cif.gz emd-30972.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30972 http://ftp.pdbj.org/pub/emdb/structures/EMD-30972 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30972 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30972 | HTTPS FTP |
-Related structure data
| Related structure data |  7e2yMC  7e2xC  7e2zC  7e32C  7e33C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30972.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30972.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Serotonin bound Serotonin 1A (5-HT1A) receptor-Gi protein complex
| Entire | Name: Serotonin bound Serotonin 1A (5-HT1A) receptor-Gi protein complex |
|---|---|
| Components |
|
-Supramolecule #1: Serotonin bound Serotonin 1A (5-HT1A) receptor-Gi protein complex
| Supramolecule | Name: Serotonin bound Serotonin 1A (5-HT1A) receptor-Gi protein complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.414047 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHASM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.915496 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD ...String: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD TTCALWDIET GQQTTTFTGH TGDVMSLSLA PDTRLFVSGA CDASAKLWDV REGMCRQTFT GHESDINAIC FF PNGNAFA TGSDDATCRL FDLRADQELM TYSHDNIICG ITSVSFSKSG RLLLAGYDDF NCNVWDALKA DRAGVLAGHD NRV SCLGVT DDGMAVATGS WDSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Soluble cytochrome b562,5-hydroxytryptamine receptor 1A
| Macromolecule | Name: Soluble cytochrome b562,5-hydroxytryptamine receptor 1A type: protein_or_peptide / ID: 4 Details: The BRIL (soluble cytochrome b562) protein was used as the expression tag Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.408512 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DYKDDDDAKL QTMHHHHHHH HHHHHHHHAD LEDNWETLND NLKVIEKADN AAQVKDALTK MRAAALDAQK ATPPKLEDKS PDSPEMKDF RHGFDILVGQ IDDALKLANE GKVKEAQAAA EQLKTTRNAY IQKYLASENL YFQGGTTTGI SDVTVSYQVI T SLLLGTLI ...String: DYKDDDDAKL QTMHHHHHHH HHHHHHHHAD LEDNWETLND NLKVIEKADN AAQVKDALTK MRAAALDAQK ATPPKLEDKS PDSPEMKDF RHGFDILVGQ IDDALKLANE GKVKEAQAAA EQLKTTRNAY IQKYLASENL YFQGGTTTGI SDVTVSYQVI T SLLLGTLI FCAVLGNACV VAAIALERSL QNVANYLIGS LAVTDLMVSV LVLPMAALYQ VLNKWTLGQV TCDLFIALDV LC CTSSIWH LCAIALDRYW AITDPIDYVN KRTPRRAAAL ISLTWLIGFL ISIPPMLGWR TPEDRSDPDA CTISKDHGYT IYS TFGAFY IPLLLMLVLY GRIFRAARFR IRKTVKKVEK TGADTRHGAS PAPQPKKSVN GESGSRNWRL GVESKAGGAL CANG AVRQG DDGAALEVIE VHRVGNSKEH LPLPSEAGPT PCAPASFERK NERNAEAKRK MALARERKTV KTLGIIMGTF ILCWL PFFI VALVLPFCES SCHMPTLLGA IINWLGYSNS LLNPVIYAYF NKDFQNAFKK IIKCKFCRQ UniProtKB: Soluble cytochrome b562, 5-hydroxytryptamine receptor 1A |
-Macromolecule #5: SEROTONIN
| Macromolecule | Name: SEROTONIN / type: ligand / ID: 5 / Number of copies: 1 / Formula: SRO |
|---|---|
| Molecular weight | Theoretical: 176.215 Da |
| Chemical component information | 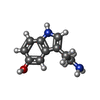 ChemComp-SRO: |
-Macromolecule #6: [(2R)-1-[oxidanyl-[(2R,3R,5S,6R)-2,3,5,6-tetrakis(oxidanyl)-4-pho...
| Macromolecule | Name: [(2R)-1-[oxidanyl-[(2R,3R,5S,6R)-2,3,5,6-tetrakis(oxidanyl)-4-phosphonooxy-cyclohexyl]oxy-phosphoryl]oxy-3-tetradecanoyloxy-propan-2-yl] (5E,8E)-hexadeca-5,8,11,14-tetraenoate type: ligand / ID: 6 / Number of copies: 1 / Formula: J40 |
|---|---|
| Molecular weight | Theoretical: 854.895 Da |
| Chemical component information | 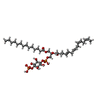 ChemComp-J40: |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 4 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 49310 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 472338 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















