[English] 日本語
 Yorodumi
Yorodumi- EMDB-23325: Cyanophycin synthetase 1 from Synechocystis sp. UTEX2470 with ADP... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23325 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cyanophycin synthetase 1 from Synechocystis sp. UTEX2470 with ADPCP and 8x(Asp-Arg)-NH2 | |||||||||
 Map data Map data | Synechocystis sp. UTEX2470 cyanophycin synthetase 1 with ADPCP and 8x(Asp-Arg)-NH2 map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationcyanophycin synthase (L-aspartate-adding) / cyanophycin synthase (L-arginine-adding) / cyanophycin synthetase activity (L-aspartate-adding) / cyanophycin synthetase activity (L-arginine-adding) / ATP binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||
 Authors Authors | Sharon I / Grogg M / Hilvert D / Schmeing TM | |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2021 Journal: Nat Chem Biol / Year: 2021Title: Structures and function of the amino acid polymerase cyanophycin synthetase. Authors: Itai Sharon / Asfarul S Haque / Marcel Grogg / Indrajit Lahiri / Dieter Seebach / Andres E Leschziner / Donald Hilvert / T Martin Schmeing /    Abstract: Cyanophycin is a natural biopolymer produced by a wide range of bacteria, consisting of a chain of poly-L-Asp residues with L-Arg residues attached to the β-carboxylate sidechains by isopeptide ...Cyanophycin is a natural biopolymer produced by a wide range of bacteria, consisting of a chain of poly-L-Asp residues with L-Arg residues attached to the β-carboxylate sidechains by isopeptide bonds. Cyanophycin is synthesized from ATP, aspartic acid and arginine by a homooligomeric enzyme called cyanophycin synthetase (CphA1). CphA1 has domains that are homologous to glutathione synthetases and muramyl ligases, but no other structural information has been available. Here, we present cryo-electron microscopy and X-ray crystallography structures of cyanophycin synthetases from three different bacteria, including cocomplex structures of CphA1 with ATP and cyanophycin polymer analogs at 2.6 Å resolution. These structures reveal two distinct tetrameric architectures, show the configuration of active sites and polymer-binding regions, indicate dynamic conformational changes and afford insight into catalytic mechanism. Accompanying biochemical interrogation of substrate binding sites, catalytic centers and oligomerization interfaces combine with the structures to provide a holistic understanding of cyanophycin biosynthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23325.map.gz emd_23325.map.gz | 230.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23325-v30.xml emd-23325-v30.xml emd-23325.xml emd-23325.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23325_fsc.xml emd_23325_fsc.xml | 13.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_23325.png emd_23325.png | 181.1 KB | ||
| Masks |  emd_23325_msk_1.map emd_23325_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Others |  emd_23325_half_map_1.map.gz emd_23325_half_map_1.map.gz emd_23325_half_map_2.map.gz emd_23325_half_map_2.map.gz | 226.3 MB 226.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23325 http://ftp.pdbj.org/pub/emdb/structures/EMD-23325 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23325 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23325 | HTTPS FTP |
-Validation report
| Summary document |  emd_23325_validation.pdf.gz emd_23325_validation.pdf.gz | 906.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23325_full_validation.pdf.gz emd_23325_full_validation.pdf.gz | 905.9 KB | Display | |
| Data in XML |  emd_23325_validation.xml.gz emd_23325_validation.xml.gz | 22.1 KB | Display | |
| Data in CIF |  emd_23325_validation.cif.gz emd_23325_validation.cif.gz | 28.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23325 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23325 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23325 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23325 | HTTPS FTP |
-Related structure data
| Related structure data |  7lgjMC  7lg5C  7lgmC  7lgnC  7lgqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23325.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23325.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Synechocystis sp. UTEX2470 cyanophycin synthetase 1 with ADPCP and 8x(Asp-Arg)-NH2 map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.855 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
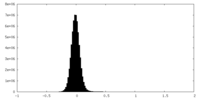
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23325_msk_1.map emd_23325_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Synechocystis sp. UTEX2470 cyanophycin synthetase 1 with ADPCP...
| File | emd_23325_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Synechocystis sp. UTEX2470 cyanophycin synthetase 1 with ADPCP and 8x(Asp-Arg)-NH2 half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Synechocystis sp. UTEX2470 cyanophycin synthetase 1 with ADPCP...
| File | emd_23325_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Synechocystis sp. UTEX2470 cyanophycin synthetase 1 with ADPCP and 8x(Asp-Arg)-NH2 half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cyanophycin synthetase 1 from Synechocystis sp. UTEX2470 with ADP...
| Entire | Name: Cyanophycin synthetase 1 from Synechocystis sp. UTEX2470 with ADPCP and 8x(Asp-Arg)-NH2 |
|---|---|
| Components |
|
-Supramolecule #1: Cyanophycin synthetase 1 from Synechocystis sp. UTEX2470 with ADP...
| Supramolecule | Name: Cyanophycin synthetase 1 from Synechocystis sp. UTEX2470 with ADPCP and 8x(Asp-Arg)-NH2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Cyanophycin synthase
| Macromolecule | Name: Cyanophycin synthase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: cyanophycin synthase (L-aspartate-adding) |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 95.758836 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKILKTLTLR GPNYWSIRRK KLIVMRLDLE DLAERPSNSI PGFYEGLIKV LPSLVEHFCS PGYQGGFLER VKEGTYMGHI VEHVALELQ ELVGMTAGFG RTRETSTPGV YNVVYEYVDE QAGRYAGRAA VRLCRSLVDT GDYPRLELEK DLEDLRDLGA N SALGPSTE ...String: MKILKTLTLR GPNYWSIRRK KLIVMRLDLE DLAERPSNSI PGFYEGLIKV LPSLVEHFCS PGYQGGFLER VKEGTYMGHI VEHVALELQ ELVGMTAGFG RTRETSTPGV YNVVYEYVDE QAGRYAGRAA VRLCRSLVDT GDYPRLELEK DLEDLRDLGA N SALGPSTE TIVTEAEARK IPWMLLSARA MVQLGYGVYQ QRIQATLSSH SGILGVELAC DKEGTKTILQ DAGIPVPRGT TI QYFDDLE EAINDVGGYP VVIKPLDGNH GRGITINVRH WQEAIAAYDL AAEESKSRAI IVERYYEGSD HRVLVVNGKL VAV AERIPA HVTGDGSSTI SELIEKTNQD PNRGDGHDNI LTKIVVNKTA IDVMERQGYN LDSVLPKDEV VYLRATANLS TGGI AIDRT DDIHPENIWL MERVAKVIGL DIAGIDVVTS DISKPLRETN GVIVEVNAAP GFRMHVAPSQ GLPRNVAAPV LDMLF PPGT PSRIPILAVT GTNGKTTTTR LLAHIYRQTG KTVGYTSTDA IYINEYCVEK GDNTGPQSAG VILRDPTVEV AVLETA RGG ILRAGLAFDS CDVGVVLNVA ADHLGLGDID TIEQMAKVKS VIAEVVDPSG YAVLNADDPL VAAMADKVKA KVAYFSM NP DNPIIQAHVR RNGIAAVYES GYLSILEGSW TLRVEQAKLI PMTMGGMAPF MIANALAACL AAFVNGLDVE VIRQGVRT F TTSAEQTPGR MNLFNLGQHH ALVDYAHNPA GYRAVGDFVK NWQGQRFGVV GGPGDRRDSD LIELGQIAAQ VFDRIIVKE DDDKRGRSEG ETADLIVKGI LQENPGASYE VILDETIALN KALDQVEEKG LVVVFPESVT RAIDLIKVRN PIGENLYFQ |
-Macromolecule #2: 8x(Asp-Arg)-NH2
| Macromolecule | Name: 8x(Asp-Arg)-NH2 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 2.186205 KDa |
| Sequence | String: (7ID)(7ID)(7ID)(7ID)(7ID)(7ID)(7ID)(7ID)(NH2) |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 12 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #4: PHOSPHOMETHYLPHOSPHONIC ACID ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOMETHYLPHOSPHONIC ACID ADENYLATE ESTER / type: ligand / ID: 4 / Number of copies: 8 / Formula: ACP |
|---|---|
| Molecular weight | Theoretical: 505.208 Da |
| Chemical component information |  ChemComp-ACP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)