+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9297 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of phosphodiesterase 6 | ||||||||||||
 Map data Map data | Cryo-EM structure of phosphodiesterase 6, primary map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | GAF domain / phosphohydrolase / G protein-coupled receptor signaling / SIGNALING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology information3',5'-cyclic-GMP phosphodiesterase / Inactivation, recovery and regulation of the phototransduction cascade / positive regulation of G protein-coupled receptor signaling pathway / Activation of the phototransduction cascade / Ca2+ pathway / positive regulation of epidermal growth factor receptor signaling pathway / photoreceptor outer segment membrane / entrainment of circadian clock by photoperiod / cGMP binding / 3',5'-cyclic-GMP phosphodiesterase activity ...3',5'-cyclic-GMP phosphodiesterase / Inactivation, recovery and regulation of the phototransduction cascade / positive regulation of G protein-coupled receptor signaling pathway / Activation of the phototransduction cascade / Ca2+ pathway / positive regulation of epidermal growth factor receptor signaling pathway / photoreceptor outer segment membrane / entrainment of circadian clock by photoperiod / cGMP binding / 3',5'-cyclic-GMP phosphodiesterase activity / 3',5'-cyclic-AMP phosphodiesterase activity / cAMP-mediated signaling / visual perception / photoreceptor disc membrane / retina development in camera-type eye / molecular adaptor activity / zinc ion binding / metal ion binding Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | ||||||||||||
 Authors Authors | Gulati S / Palczewski K / Kovacik L / Stahlberg H | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2019 Journal: Sci Adv / Year: 2019Title: Cryo-EM structure of phosphodiesterase 6 reveals insights into the allosteric regulation of type I phosphodiesterases. Authors: Sahil Gulati / Krzysztof Palczewski / Andreas Engel / Henning Stahlberg / Lubomir Kovacik /   Abstract: Cyclic nucleotide phosphodiesterases (PDEs) work in conjunction with adenylate/guanylate cyclases to regulate the key second messengers of G protein-coupled receptor signaling. Previous attempts to ...Cyclic nucleotide phosphodiesterases (PDEs) work in conjunction with adenylate/guanylate cyclases to regulate the key second messengers of G protein-coupled receptor signaling. Previous attempts to determine the full-length structure of PDE family members at high-resolution have been hindered by structural flexibility, especially in their linker regions and N- and C-terminal ends. Therefore, most structure-activity relationship studies have so far focused on truncated and conserved catalytic domains rather than the regulatory domains that allosterically govern the activity of most PDEs. Here, we used single-particle cryo-electron microscopy to determine the structure of the full-length PDE6αβ2γ complex. The final density map resolved at 3.4 Å reveals several previously unseen structural features, including a coiled N-terminal domain and the interface of PDE6γ subunits with the PDE6αβ heterodimer. Comparison of the PDE6αβ2γ complex with the closed state of PDE2A sheds light on the conformational changes associated with the allosteric activation of type I PDEs. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9297.map.gz emd_9297.map.gz | 1.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9297-v30.xml emd-9297-v30.xml emd-9297.xml emd-9297.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9297.png emd_9297.png | 157.1 KB | ||
| Masks |  emd_9297_msk_1.map emd_9297_msk_1.map | 27 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-9297.cif.gz emd-9297.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9297 http://ftp.pdbj.org/pub/emdb/structures/EMD-9297 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9297 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9297 | HTTPS FTP |
-Validation report
| Summary document |  emd_9297_validation.pdf.gz emd_9297_validation.pdf.gz | 422.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9297_full_validation.pdf.gz emd_9297_full_validation.pdf.gz | 422 KB | Display | |
| Data in XML |  emd_9297_validation.xml.gz emd_9297_validation.xml.gz | 5.8 KB | Display | |
| Data in CIF |  emd_9297_validation.cif.gz emd_9297_validation.cif.gz | 6.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9297 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9297 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9297 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9297 | HTTPS FTP |
-Related structure data
| Related structure data |  6mzbMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10228 (Title: Cryo-EM structure of phosphodiesterase 6 reveals insights into the allosteric regulation of type I phosphodiesterases EMPIAR-10228 (Title: Cryo-EM structure of phosphodiesterase 6 reveals insights into the allosteric regulation of type I phosphodiesterasesData size: 166.3 Data #1: Aligned micrographs of Phosphodiesterase 6 [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9297.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9297.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of phosphodiesterase 6, primary map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.058 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
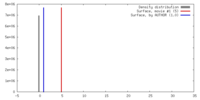
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_9297_msk_1.map emd_9297_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
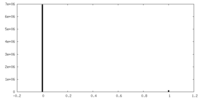
| Projections & Slices |
| ||||||||||||
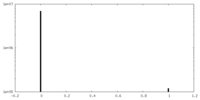
| Density Histograms |
- Sample components
Sample components
-Entire : Phosphodiesterase 6
| Entire | Name: Phosphodiesterase 6 |
|---|---|
| Components |
|
-Supramolecule #1: Phosphodiesterase 6
| Supramolecule | Name: Phosphodiesterase 6 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta
| Macromolecule | Name: Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: 3',5'-cyclic-GMP phosphodiesterase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 98.449648 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSLSEGQVHR FLDQNPGFAD QYFGRKLSPE DVANACEDGC PEGCTSFREL CQVEESAALF ELVQDMQENV NMERVVFKIL RRLCSILHA DRCSLFMYRQ RNGVAELATR LFSVQPDSVL EDCLVPPDSE IVFPLDIGVV GHVAQTKKMV NVQDVMECPH F SSFADELT ...String: MSLSEGQVHR FLDQNPGFAD QYFGRKLSPE DVANACEDGC PEGCTSFREL CQVEESAALF ELVQDMQENV NMERVVFKIL RRLCSILHA DRCSLFMYRQ RNGVAELATR LFSVQPDSVL EDCLVPPDSE IVFPLDIGVV GHVAQTKKMV NVQDVMECPH F SSFADELT DYVTRNILAT PIMNGKDVVA VIMAVNKLDG PCFTSEDEDV FLKYLNFGTL NLKIYHLSYL HNCETRRGQV LL WSANKVF EELTDIERQF HKAFYTVRAY LNCDRYSVGL LDMTKEKEFF DVWPVLMGEA QAYSGPRTPD GREILFYKVI DYI LHGKED IKVIPSPPAD HWALASGLPT YVAESGFICN IMNAPADEMF NFQEGPLDDS GWIVKNVLSM PIVNKKEEIV GVAT FYNRK DGKPFDEQDE VLMESLTQFL GWSVLNTDTY DKMNKLENRK DIAQDMVLYH VRCDREEIQL ILPTRERLGK EPADC EEDE LGKILKEVLP GPAKFDIYEF HFSDLECTEL ELVKCGIQMY YELGVVRKFQ IPQEVLVRFL FSVSKGYRRI TYHNWR HGF NVAQTMFTLL MTGKLKSYYT DLEAFAMVTA GLCHDIDHRG TNNLYQMKSQ NPLAKLHGSS ILERHHLEFG KFLLSEE TL NIYQNLNRRQ HEHVIHLMDI AIIATDLALY FKKRTMFQKI VDESKNYEDR KSWVEYLSLE TTRKEIVMAM MMTACDLS A ITKPWEVQSK VALLVAAEFW EQGDLERTVL DQQPIPMMDR NKAAELPKLQ VGFIDFVCTF VYKEFSRFHE EILPMFDRL QNNRKEWKAL ADEYEAKVKA LEEDQKKETT AKKVGTEICN GGPAPRSSTC RIL UniProtKB: Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta |
-Macromolecule #2: Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha
| Macromolecule | Name: Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: 3',5'-cyclic-GMP phosphodiesterase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 99.461789 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGEVTAEEVE KFLDSNVSFA KQYYNLRYRA KVISDLLGPR EAAVDFSNYH ALNSVEESEI IFDLLRDFQD NLQAEKCVFN VMKKLCFLL QADRMSLFMY RARNGIAELA TRLFNVHKDA VLEECLVAPD SEIVFPLDMG VVGHVALSKK IVNVPNTEED E HFCDFVDT ...String: MGEVTAEEVE KFLDSNVSFA KQYYNLRYRA KVISDLLGPR EAAVDFSNYH ALNSVEESEI IFDLLRDFQD NLQAEKCVFN VMKKLCFLL QADRMSLFMY RARNGIAELA TRLFNVHKDA VLEECLVAPD SEIVFPLDMG VVGHVALSKK IVNVPNTEED E HFCDFVDT LTEYQTKNIL ASPIMNGKDV VAIIMVVNKV DGPHFTENDE EILLKYLNFA NLIMKVFHLS YLHNCETRRG QI LLWSGSK VFEELTDIER QFHKALYTVR AFLNCDRYSV GLLDMTKQKE FFDVWPVLMG EAPPYAGPRT PDGREINFYK VID YILHGK EDIKVIPNPP PDHWALVSGL PTYVAQNGLI CNIMNAPSED FFAFQKEPLD ESGWMIKNVL SMPIVNKKEE IVGV ATFYN RKDGKPFDEM DETLMESLTQ FLGWSVLNPD TYELMNKLEN RKDIFQDMVK YHVKCDNEEI QTILKTREVY GKEPW ECEE EELAEILQGE LPDADKYEIN KFHFSDLPLT ELELVKCGIQ MYYELKVVDK FHIPQEALVR FMYSLSKGYR RITYHN WRH GFNVGQTMFS LLVTGKLKRY FTDLEALAMV TAAFCHDIDH RGTNNLYQMK SQNPLAKLHG SSILERHHLE FGKTLLR DE SLNIFQNLNR RQHEHAIHMM DIAIIATDLA LYFKKRTMFQ KIVDQSKTYE TQQEWTQYMM LDQTRKEIVM AMMMTACD L SAITKPWEVQ SKVALLVAAE FWEQGDLERT VLQQNPIPMM DRNKADELPK LQVGFIDFVC TFVYKEFSRF HEEITPMLD GITNNRKEWK ALADEYETKM KGLEEEKQKQ QAANQAAAGS QHGGKQPGGG PASKSCCVQ UniProtKB: Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha |
-Macromolecule #3: Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiestera...
| Macromolecule | Name: Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit gamma type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO / EC number: 3',5'-cyclic-GMP phosphodiesterase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 9.684229 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNLEPPKAEI RSATRVMGGP VTPRKGPPKF KQRQTRQFKS KPPKKGVQGF GDDIPGMEGL GTDITVICPW EAFNHLELHE LAQYGII UniProtKB: Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit gamma |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: GUANOSINE-3',5'-MONOPHOSPHATE
| Macromolecule | Name: GUANOSINE-3',5'-MONOPHOSPHATE / type: ligand / ID: 6 / Number of copies: 2 / Formula: 35G |
|---|---|
| Molecular weight | Theoretical: 345.205 Da |
| Chemical component information |  ChemComp-35G: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 43597 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)