[English] 日本語
 Yorodumi
Yorodumi- EMDB-9981: Local map of the Rd region of the phycobilisome from the red alga... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9981 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Local map of the Rd region of the phycobilisome from the red alga P. purpureum | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Porphyridium purpureum (eukaryote) Porphyridium purpureum (eukaryote) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.82 Å | |||||||||
 Authors Authors | Sui SF / Ma JF / You X / Sun S | |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Authors: Jianfei Ma / Xin You / Shan Sun / Xiaoxiao Wang / Song Qin / Sen-Fang Sui /  Abstract: Photosynthetic organisms have developed various light-harvesting systems to adapt to their environments. Phycobilisomes are large light-harvesting protein complexes found in cyanobacteria and red ...Photosynthetic organisms have developed various light-harvesting systems to adapt to their environments. Phycobilisomes are large light-harvesting protein complexes found in cyanobacteria and red algae, although how the energies of the chromophores within these complexes are modulated by their environment is unclear. Here we report the cryo-electron microscopy structure of a 14.7-megadalton phycobilisome with a hemiellipsoidal shape from the red alga Porphyridium purpureum. Within this complex we determine the structures of 706 protein subunits, including 528 phycoerythrin, 72 phycocyanin, 46 allophycocyanin and 60 linker proteins. In addition, 1,598 chromophores are resolved comprising 1,430 phycoerythrobilin, 48 phycourobilin and 120 phycocyanobilin molecules. The markedly improved resolution of our structure compared with that of the phycobilisome of Griffithsia pacifica enabled us to build an accurate atomic model of the P. purpureum phycobilisome system. The model reveals how the linker proteins affect the microenvironment of the chromophores, and suggests that interactions of the aromatic amino acids of the linker proteins with the chromophores may be a key factor in fine-tuning the energy states of the chromophores to ensure the efficient unidirectional transfer of energy. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9981.map.gz emd_9981.map.gz | 40.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9981-v30.xml emd-9981-v30.xml emd-9981.xml emd-9981.xml | 10.8 KB 10.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9981.png emd_9981.png | 17.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9981 http://ftp.pdbj.org/pub/emdb/structures/EMD-9981 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9981 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9981 | HTTPS FTP |
-Validation report
| Summary document |  emd_9981_validation.pdf.gz emd_9981_validation.pdf.gz | 78.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9981_full_validation.pdf.gz emd_9981_full_validation.pdf.gz | 77.6 KB | Display | |
| Data in XML |  emd_9981_validation.xml.gz emd_9981_validation.xml.gz | 493 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9981 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9981 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9981 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9981 | HTTPS FTP |
-Related structure data
| Related structure data |  9976C  9977C  9978C  9979C  9980C  9982C  9983C  9984C  9985C  9986C  9987C  9988C  6kgxC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9981.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9981.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.091 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
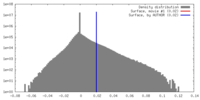
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Local map of the Rd region of the phycobilisome from the red alga...
| Entire | Name: Local map of the Rd region of the phycobilisome from the red alga P. purpureum |
|---|---|
| Components |
|
-Supramolecule #1: Local map of the Rd region of the phycobilisome from the red alga...
| Supramolecule | Name: Local map of the Rd region of the phycobilisome from the red alga P. purpureum type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Porphyridium purpureum (eukaryote) Porphyridium purpureum (eukaryote) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL | ||||||
|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
| ||||||
| Sugar embedding | Material: ice | ||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-32 / Number real images: 16218 / Average exposure time: 5.6 sec. / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)