[English] 日本語
 Yorodumi
Yorodumi- EMDB-9103: The D1 and D2 domain rings of NSF engaging the SNAP-25 N-terminus... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9103 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The D1 and D2 domain rings of NSF engaging the SNAP-25 N-terminus within the 20S supercomplex (focused refinement on D1/D2 rings, class 2) | |||||||||
 Map data Map data | The unsharpened map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SNARE / NSF / SNAP / ATPase / AAA / disassembly / synapse / membrane fusion / exocytosis / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationBLOC-1 complex / synaptic vesicle fusion to presynaptic active zone membrane / Other interleukin signaling / synaptobrevin 2-SNAP-25-syntaxin-1a-complexin II complex / synaptobrevin 2-SNAP-25-syntaxin-1a complex / presynaptic dense core vesicle exocytosis / synaptobrevin 2-SNAP-25-syntaxin-1a-complexin I complex / extrinsic component of presynaptic membrane / calcium ion-regulated exocytosis of neurotransmitter / Glutamate Neurotransmitter Release Cycle ...BLOC-1 complex / synaptic vesicle fusion to presynaptic active zone membrane / Other interleukin signaling / synaptobrevin 2-SNAP-25-syntaxin-1a-complexin II complex / synaptobrevin 2-SNAP-25-syntaxin-1a complex / presynaptic dense core vesicle exocytosis / synaptobrevin 2-SNAP-25-syntaxin-1a-complexin I complex / extrinsic component of presynaptic membrane / calcium ion-regulated exocytosis of neurotransmitter / Glutamate Neurotransmitter Release Cycle / Norepinephrine Neurotransmitter Release Cycle / Acetylcholine Neurotransmitter Release Cycle / Serotonin Neurotransmitter Release Cycle / GABA synthesis, release, reuptake and degradation / Dopamine Neurotransmitter Release Cycle / SNARE complex disassembly / regulation of establishment of protein localization / ribbon synapse / SNARE complex / SNAP receptor activity / ATP-dependent protein disaggregase activity / positive regulation of hormone secretion / intra-Golgi vesicle-mediated transport / Golgi to plasma membrane protein transport / Golgi stack / neurotransmitter secretion / vesicle-fusing ATPase / syntaxin-1 binding / endosomal transport / Neutrophil degranulation / SNARE complex assembly / regulation of synapse assembly / myosin binding / regulation of neuron projection development / synaptic vesicle priming / exocytosis / positive regulation of receptor recycling / associative learning / synaptic vesicle exocytosis / voltage-gated potassium channel activity / long-term memory / axonal growth cone / somatodendritic compartment / voltage-gated potassium channel complex / photoreceptor inner segment / ionotropic glutamate receptor binding / axonogenesis / SNARE binding / PDZ domain binding / filopodium / intracellular protein transport / locomotory behavior / trans-Golgi network / positive regulation of insulin secretion / potassium ion transport / long-term synaptic potentiation / terminal bouton / neuron differentiation / calcium-dependent protein binding / synaptic vesicle / positive regulation of protein catabolic process / actin cytoskeleton / lamellipodium / growth cone / presynaptic membrane / midbody / cell cortex / vesicle / transmembrane transporter binding / cytoskeleton / endosome / neuron projection / protein domain specific binding / axon / neuronal cell body / synapse / lipid binding / protein kinase binding / protein-containing complex binding / perinuclear region of cytoplasm / glutamatergic synapse / ATP hydrolysis activity / ATP binding / metal ion binding / identical protein binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
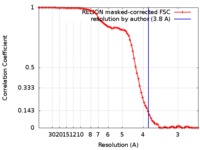
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | White KI / Zhao M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2018 Journal: Elife / Year: 2018Title: Structural principles of SNARE complex recognition by the AAA+ protein NSF. Authors: K Ian White / Minglei Zhao / Ucheor B Choi / Richard A Pfuetzner / Axel T Brunger /  Abstract: The recycling of SNARE proteins following complex formation and membrane fusion is an essential process in eukaryotic trafficking. A highly conserved AAA+ protein, NSF (-ethylmaleimide sensitive ...The recycling of SNARE proteins following complex formation and membrane fusion is an essential process in eukaryotic trafficking. A highly conserved AAA+ protein, NSF (-ethylmaleimide sensitive factor) and an adaptor protein, SNAP (soluble NSF attachment protein), disassemble the SNARE complex. We report electron-cryomicroscopy structures of the complex of NSF, αSNAP, and the full-length soluble neuronal SNARE complex (composed of syntaxin-1A, synaptobrevin-2, SNAP-25A) in the presence of ATP under non-hydrolyzing conditions at ~3.9 Å resolution. These structures reveal electrostatic interactions by which two αSNAP molecules interface with a specific surface of the SNARE complex. This interaction positions the SNAREs such that the 15 N-terminal residues of SNAP-25A are loaded into the D1 ring pore of NSF via a spiral pattern of interactions between a conserved tyrosine NSF residue and SNAP-25A backbone atoms. This loading process likely precedes ATP hydrolysis. Subsequent ATP hydrolysis then drives complete disassembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9103.map.gz emd_9103.map.gz | 35.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9103-v30.xml emd-9103-v30.xml emd-9103.xml emd-9103.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9103_fsc.xml emd_9103_fsc.xml | 8.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_9103.png emd_9103.png | 31.2 KB | ||
| Filedesc metadata |  emd-9103.cif.gz emd-9103.cif.gz | 7 KB | ||
| Others |  emd_9103_additional.map.gz emd_9103_additional.map.gz | 43.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9103 http://ftp.pdbj.org/pub/emdb/structures/EMD-9103 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9103 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9103 | HTTPS FTP |
-Related structure data
| Related structure data |  6mdpMC  9100C  9101C  9102C  6mdmC  6mdnC  6mdoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9103.map.gz / Format: CCP4 / Size: 46.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9103.map.gz / Format: CCP4 / Size: 46.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The unsharpened map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
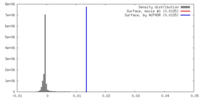
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: The sharpened map.
| File | emd_9103_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The sharpened map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
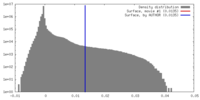
| Density Histograms |
- Sample components
Sample components
-Entire : 20S supercomplex consisting of soluble neuronal SNARE complex, al...
| Entire | Name: 20S supercomplex consisting of soluble neuronal SNARE complex, alpha-SNAP, and N-ethylmaleimide sensitive factor (NSF) |
|---|---|
| Components |
|
-Supramolecule #1: 20S supercomplex consisting of soluble neuronal SNARE complex, al...
| Supramolecule | Name: 20S supercomplex consisting of soluble neuronal SNARE complex, alpha-SNAP, and N-ethylmaleimide sensitive factor (NSF) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Molecular weight | Theoretical: 23.625 KDa |
-Supramolecule #2: N-ethylmaleimide sensitive factor
| Supramolecule | Name: N-ethylmaleimide sensitive factor / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Synaptosomal-associated protein 25
| Supramolecule | Name: Synaptosomal-associated protein 25 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Vesicle-fusing ATPase
| Macromolecule | Name: Vesicle-fusing ATPase / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: vesicle-fusing ATPase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 85.509227 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGHHHHHHDY DIPTTENLYF QGAHMAGRSM QAARCPTDEL SLSNCAVVSE KDYQSGQHVI VRTSPNHKYI FTLRTHPSVV PGSVAFSLP QRKWAGLSIG QEIEVALYSF DKAKQCIGTM TIEIDFLQKK NIDSNPYDTD KMAAEFIQQF NNQAFSVGQQ L VFSFNDKL ...String: MGHHHHHHDY DIPTTENLYF QGAHMAGRSM QAARCPTDEL SLSNCAVVSE KDYQSGQHVI VRTSPNHKYI FTLRTHPSVV PGSVAFSLP QRKWAGLSIG QEIEVALYSF DKAKQCIGTM TIEIDFLQKK NIDSNPYDTD KMAAEFIQQF NNQAFSVGQQ L VFSFNDKL FGLLVKDIEA MDPSILKGEP ASGKRQKIEV GLVVGNSQVA FEKAENSSLN LIGKAKTKEN RQSIINPDWN FE KMGIGGL DKEFSDIFRR AFASRVFPPE IVEQMGCKHV KGILLYGPPG CGKTLLARQI GKMLNAREPK VVNGPEILNK YVG ESEANI RKLFADAEEE QRRLGANSGL HIIIFDEIDA ICKQRGSMAG STGVHDTVVN QLLSKIDGVE QLNNILVIGM TNRP DLIDE ALLRPGRLEV KMEIGLPDEK GRLQILHIHT ARMRGHQLLS ADVDIKELAV ETKNFSGAEL EGLVRAAQST AMNRH IIAS TKVEVDMEKA ESLQVTRGDF LASLENDIKP AFGTNQEDYA SYIMNGIIKW GDPVTRVLDD GELLVQQTKN SDRTPL VSV LLEGPPHSGK TALAAKIAEE SNFPFIKICS PDKMIGFSET AKCQAMKKIF DDAYKSQLSC VVVDDIERLL DYVPIGP RF SNLVLQALLV LLKKAPPQGR KLLIIGTTSR KDVLQEMEML NAFSTTIHVP NIATGEQLLE ALELLGNFKD KERTTIAQ Q VKGKKVWIGI KKLLMLIEMS LQMDPEYRVR KFLALLREEG ASPLDFD UniProtKB: Vesicle-fusing ATPase |
-Macromolecule #2: Synaptosomal-associated protein 25
| Macromolecule | Name: Synaptosomal-associated protein 25 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.512387 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASMAEDADM RNELEEMQRR ADQLADESLE STRRMLQLVE ESKDAGIRTL VMLDEQGEQL DRVEEGMNHI NQDMKEAEKN LKDLGKCCG LFICPCNKLK SSDAYKKAWG NNQDGVVASQ PARVVDEREQ MAISGGFIRR VTNDARENEM DENLEQVSGI I GNLRHMAL ...String: MASMAEDADM RNELEEMQRR ADQLADESLE STRRMLQLVE ESKDAGIRTL VMLDEQGEQL DRVEEGMNHI NQDMKEAEKN LKDLGKCCG LFICPCNKLK SSDAYKKAWG NNQDGVVASQ PARVVDEREQ MAISGGFIRR VTNDARENEM DENLEQVSGI I GNLRHMAL DMGNEIDTQN RQIDRIMEKA DSNKTRIDEA NQRATKMLG UniProtKB: Synaptosomal-associated protein 25 |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 9 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 15 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK I / Details: Blot for 3.5 seconds before plunging.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 2-40 / Number grids imaged: 2 / Number real images: 5418 / Average exposure time: 10.0 sec. / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-6mdp: |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)