[English] 日本語
 Yorodumi
Yorodumi- EMDB-8335: Structural basis for dynamic regulation of the human 26S proteasome -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8335 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural basis for dynamic regulation of the human 26S proteasome | |||||||||
 Map data Map data | The half 26S proteasome reconstruction in the SB state focusing on the regulatory particle from 18443 particles of half 26S | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of inclusion body assembly / Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / thyrotropin-releasing hormone receptor binding / modulation by host of viral transcription / meiosis I / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / proteasome accessory complex / integrator complex / purine ribonucleoside triphosphate binding ...positive regulation of inclusion body assembly / Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / thyrotropin-releasing hormone receptor binding / modulation by host of viral transcription / meiosis I / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / proteasome accessory complex / integrator complex / purine ribonucleoside triphosphate binding / proteasome regulatory particle / positive regulation of proteasomal protein catabolic process / cytosolic proteasome complex / proteasome regulatory particle, lid subcomplex / proteasome-activating activity / proteasome regulatory particle, base subcomplex / metal-dependent deubiquitinase activity / negative regulation of programmed cell death / regulation of endopeptidase activity / protein K63-linked deubiquitination / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / Regulation of ornithine decarboxylase (ODC) / proteasome core complex / Resolution of D-loop Structures through Holliday Junction Intermediates / Cross-presentation of soluble exogenous antigens (endosomes) / : / K63-linked deubiquitinase activity / Somitogenesis / Impaired BRCA2 binding to RAD51 / myofibril / immune system process / proteasome binding / transcription factor binding / regulation of protein catabolic process / proteasome storage granule / Presynaptic phase of homologous DNA pairing and strand exchange / blastocyst development / general transcription initiation factor binding / polyubiquitin modification-dependent protein binding / NF-kappaB binding / endopeptidase activator activity / protein deubiquitination / proteasome assembly / positive regulation of RNA polymerase II transcription preinitiation complex assembly / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / proteasome core complex, alpha-subunit complex / threonine-type endopeptidase activity / mRNA export from nucleus / enzyme regulator activity / regulation of proteasomal protein catabolic process / SARS-CoV-1 targets host intracellular signalling and regulatory pathways / ERAD pathway / inclusion body / sarcomere / proteasome complex / Regulation of activated PAK-2p34 by proteasome mediated degradation / ciliary basal body / proteolysis involved in protein catabolic process / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / Autodegradation of Cdh1 by Cdh1:APC/C / APC/C:Cdc20 mediated degradation of Securin / SCF-beta-TrCP mediated degradation of Emi1 / Asymmetric localization of PCP proteins / NIK-->noncanonical NF-kB signaling / Ubiquitin-dependent degradation of Cyclin D / AUF1 (hnRNP D0) binds and destabilizes mRNA / TNFR2 non-canonical NF-kB pathway / stem cell differentiation / Vpu mediated degradation of CD4 / Assembly of the pre-replicative complex / Degradation of DVL / negative regulation of inflammatory response to antigenic stimulus / Ubiquitin Mediated Degradation of Phosphorylated Cdc25A / Dectin-1 mediated noncanonical NF-kB signaling / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / lipopolysaccharide binding / Hh mutants are degraded by ERAD / Degradation of AXIN / Activation of NF-kappaB in B cells / Degradation of GLI1 by the proteasome / Hedgehog ligand biogenesis / Defective CFTR causes cystic fibrosis / G2/M Checkpoints / Negative regulation of NOTCH4 signaling / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Autodegradation of the E3 ubiquitin ligase COP1 / Vif-mediated degradation of APOBEC3G / double-strand break repair via homologous recombination / P-body / Hedgehog 'on' state / Regulation of RUNX3 expression and activity / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / MAPK6/MAPK4 signaling Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 6.8 Å | |||||||||
 Authors Authors | Chen S / Wu J / Lu Y / Ma YB / Lee BH / Yu Z / Ouyang Q / Finley D / Kirschner MW / Mao Y | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2016 Journal: Proc Natl Acad Sci U S A / Year: 2016Title: Structural basis for dynamic regulation of the human 26S proteasome. Authors: Shuobing Chen / Jiayi Wu / Ying Lu / Yong-Bei Ma / Byung-Hoon Lee / Zhou Yu / Qi Ouyang / Daniel J Finley / Marc W Kirschner / Youdong Mao /   Abstract: The proteasome is the major engine of protein degradation in all eukaryotic cells. At the heart of this machine is a heterohexameric ring of AAA (ATPases associated with diverse cellular activities) ...The proteasome is the major engine of protein degradation in all eukaryotic cells. At the heart of this machine is a heterohexameric ring of AAA (ATPases associated with diverse cellular activities) proteins that unfolds ubiquitylated target proteins that are concurrently translocated into a proteolytic chamber and degraded into peptides. Using cryoelectron microscopy, we determined a near-atomic-resolution structure of the 2.5-MDa human proteasome in its ground state, as well as subnanometer-resolution structures of the holoenzyme in three alternative conformational states. The substrate-unfolding AAA-ATPase channel is narrowed by 10 inward-facing pore loops arranged into two helices that run in parallel with each other, one hydrophobic in character and the other highly charged. The gate of the core particle was unexpectedly found closed in the ground state and open in only one of the alternative states. Coordinated, stepwise conformational changes of the regulatory particle couple ATP hydrolysis to substrate translocation and regulate gating of the core particle, leading to processive degradation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8335.map.gz emd_8335.map.gz | 161.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8335-v30.xml emd-8335-v30.xml emd-8335.xml emd-8335.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8335.png emd_8335.png | 63 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8335 http://ftp.pdbj.org/pub/emdb/structures/EMD-8335 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8335 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8335 | HTTPS FTP |
-Validation report
| Summary document |  emd_8335_validation.pdf.gz emd_8335_validation.pdf.gz | 386.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8335_full_validation.pdf.gz emd_8335_full_validation.pdf.gz | 386.2 KB | Display | |
| Data in XML |  emd_8335_validation.xml.gz emd_8335_validation.xml.gz | 6.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8335 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8335 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8335 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8335 | HTTPS FTP |
-Related structure data
| Related structure data |  5t0hMC  8332C  8333C  8334C  8336C  8337C  5t0cC  5t0gC  5t0iC  5t0jC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10072 (Title: Structural basis for dynamic regulation of the human 26S proteasome EMPIAR-10072 (Title: Structural basis for dynamic regulation of the human 26S proteasomeData size: 2.0 TB Data #1: Drift-corrected micrographs of human 26S proteasome holoenzyme [micrographs - single frame] Data #2: Classified particle datasets for the human proteasome in four conformational states [picked particles - multiframe - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8335.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8335.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The half 26S proteasome reconstruction in the SB state focusing on the regulatory particle from 18443 particles of half 26S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
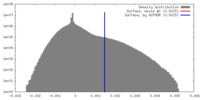
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : 26S proteasome holoenzyme
| Entire | Name: 26S proteasome holoenzyme |
|---|---|
| Components |
|
-Supramolecule #1: 26S proteasome holoenzyme
| Supramolecule | Name: 26S proteasome holoenzyme / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Experimental: 2.5 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
| Grid | Model: C-Flat R1/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Support film - Film thickness: 50.0 nm / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blotted for 2 seconds, blotting force 3. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 7420 pixel / Digitization - Dimensions - Height: 7676 pixel / Digitization - Sampling interval: 5.0 µm / Digitization - Frames/image: 3-20 / Number grids imaged: 12 / Number real images: 10367 / Average exposure time: 9.0 sec. / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 28736 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -3.0 µm / Nominal defocus min: -1.0 µm / Nominal magnification: 21000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 150 |
|---|---|
| Output model |  PDB-5t0h: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)