[English] 日本語
 Yorodumi
Yorodumi- EMDB-8107: Structure of RelA bound to the 70S ribosome (overall map, class 1) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8107 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of RelA bound to the 70S ribosome (overall map, class 1) | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ribosome / tRNA / RelA / ppGpp | |||||||||
| Function / homology |  Function and homology information Function and homology informationguanosine tetraphosphate metabolic process / guanosine-3',5'-bis(diphosphate) 3'-diphosphatase activity / GTP diphosphokinase / GTP diphosphokinase activity / guanosine tetraphosphate biosynthetic process / nucleobase-containing small molecule interconversion / negative regulation of cytoplasmic translational initiation / response to starvation / stringent response / ornithine decarboxylase inhibitor activity ...guanosine tetraphosphate metabolic process / guanosine-3',5'-bis(diphosphate) 3'-diphosphatase activity / GTP diphosphokinase / GTP diphosphokinase activity / guanosine tetraphosphate biosynthetic process / nucleobase-containing small molecule interconversion / negative regulation of cytoplasmic translational initiation / response to starvation / stringent response / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / positive regulation of ribosome biogenesis / negative regulation of cytoplasmic translation / translational termination / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / negative regulation of translational initiation / regulation of mRNA stability / mRNA regulatory element binding translation repressor activity / ribosome assembly / assembly of large subunit precursor of preribosome / positive regulation of RNA splicing / transcription elongation factor complex / cytosolic ribosome assembly / regulation of DNA-templated transcription elongation / DNA endonuclease activity / response to reactive oxygen species / transcription antitermination / regulation of cell growth / translational initiation / DNA-templated transcription termination / maintenance of translational fidelity / response to radiation / mRNA 5'-UTR binding / ribosomal small subunit biogenesis / small ribosomal subunit rRNA binding / large ribosomal subunit / ribosome biogenesis / ribosome binding / kinase activity / regulation of translation / ribosomal small subunit assembly / small ribosomal subunit / 5S rRNA binding / large ribosomal subunit rRNA binding / transferase activity / cytosolic small ribosomal subunit / ribosomal large subunit assembly / cytoplasmic translation / cytosolic large ribosomal subunit / tRNA binding / molecular adaptor activity / negative regulation of translation / rRNA binding / ribosome / structural constituent of ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / GTP binding / DNA binding / RNA binding / zinc ion binding / ATP binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
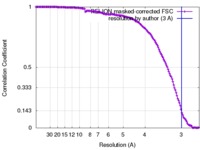
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Brown A / Fernandez IS | |||||||||
 Citation Citation |  Journal: Nature / Year: 2016 Journal: Nature / Year: 2016Title: Ribosome-dependent activation of stringent control. Authors: Alan Brown / Israel S Fernández / Yuliya Gordiyenko / V Ramakrishnan /  Abstract: In order to survive, bacteria continually sense, and respond to, environmental fluctuations. Stringent control represents a key bacterial stress response to nutrient starvation that leads to rapid ...In order to survive, bacteria continually sense, and respond to, environmental fluctuations. Stringent control represents a key bacterial stress response to nutrient starvation that leads to rapid and comprehensive reprogramming of metabolic and transcriptional patterns. In general, transcription of genes for growth and proliferation is downregulated, while those important for survival and virulence are upregulated. Amino acid starvation is sensed by depletion of the aminoacylated tRNA pools, and this results in accumulation of ribosomes stalled with non-aminoacylated (uncharged) tRNA in the ribosomal A site. RelA is recruited to stalled ribosomes and activated to synthesize a hyperphosphorylated guanosine analogue, (p)ppGpp, which acts as a pleiotropic secondary messenger. However, structural information about how RelA recognizes stalled ribosomes and discriminates against aminoacylated tRNAs is missing. Here we present the cryo-electron microscopy structure of RelA bound to the bacterial ribosome stalled with uncharged tRNA. The structure reveals that RelA utilizes a distinct binding site compared to the translational factors, with a multi-domain architecture that wraps around a highly distorted A-site tRNA. The TGS (ThrRS, GTPase and SpoT) domain of RelA binds the CCA tail to orient the free 3' hydroxyl group of the terminal adenosine towards a β-strand, such that an aminoacylated tRNA at this position would be sterically precluded. The structure supports a model in which association of RelA with the ribosome suppresses auto-inhibition to activate synthesis of (p)ppGpp and initiate the stringent response. Since stringent control is responsible for the survival of pathogenic bacteria under stress conditions, and contributes to chronic infections and antibiotic tolerance, RelA represents a good target for the development of novel antibacterial therapeutics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8107.map.gz emd_8107.map.gz | 23.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8107-v30.xml emd-8107-v30.xml emd-8107.xml emd-8107.xml | 77.9 KB 77.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8107_fsc.xml emd_8107_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_8107.png emd_8107.png | 173.2 KB | ||
| Filedesc metadata |  emd-8107.cif.gz emd-8107.cif.gz | 15.4 KB | ||
| Others |  emd_8107_half_map_1.map.gz emd_8107_half_map_1.map.gz emd_8107_half_map_2.map.gz emd_8107_half_map_2.map.gz | 193.5 MB 193.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8107 http://ftp.pdbj.org/pub/emdb/structures/EMD-8107 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8107 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8107 | HTTPS FTP |
-Validation report
| Summary document |  emd_8107_validation.pdf.gz emd_8107_validation.pdf.gz | 735.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8107_full_validation.pdf.gz emd_8107_full_validation.pdf.gz | 735.5 KB | Display | |
| Data in XML |  emd_8107_validation.xml.gz emd_8107_validation.xml.gz | 22.1 KB | Display | |
| Data in CIF |  emd_8107_validation.cif.gz emd_8107_validation.cif.gz | 29.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8107 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8107 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8107 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8107 | HTTPS FTP |
-Related structure data
| Related structure data |  5iqrMC  8108C  8109C  8110C  8111C  8112C  8113C  8114C  8115C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8107.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8107.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.34 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: None
| File | emd_8107_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: None
| File | emd_8107_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : RIBOSOME
+Supramolecule #1: RIBOSOME
+Macromolecule #1: 50S ribosomal protein L2
+Macromolecule #2: 50S ribosomal protein L3
+Macromolecule #3: 50S ribosomal protein L4
+Macromolecule #4: 50S ribosomal protein L5
+Macromolecule #5: 50S ribosomal protein L6
+Macromolecule #6: 50S ribosomal protein L9
+Macromolecule #7: 50S ribosomal protein L10
+Macromolecule #8: 50S ribosomal protein L11
+Macromolecule #9: 50S ribosomal protein L13
+Macromolecule #10: 50S ribosomal protein L14
+Macromolecule #11: 50S ribosomal protein L15
+Macromolecule #12: 50S ribosomal protein L16
+Macromolecule #13: 50S ribosomal protein L17
+Macromolecule #14: 50S ribosomal protein L18
+Macromolecule #15: 50S ribosomal protein L19
+Macromolecule #16: 50S ribosomal protein L20
+Macromolecule #17: 50S ribosomal protein L21
+Macromolecule #18: 50S ribosomal protein L22
+Macromolecule #19: 50S ribosomal protein L23
+Macromolecule #20: 50S ribosomal protein L24
+Macromolecule #21: 50S ribosomal protein L25
+Macromolecule #22: 50S ribosomal protein L27
+Macromolecule #23: 50S ribosomal protein L28
+Macromolecule #24: 50S ribosomal protein L29
+Macromolecule #25: 50S ribosomal protein L30
+Macromolecule #26: 50S ribosomal protein L31
+Macromolecule #27: 50S ribosomal protein L32
+Macromolecule #28: 50S ribosomal protein L33
+Macromolecule #29: 50S ribosomal protein L34
+Macromolecule #30: 50S ribosomal protein L35
+Macromolecule #31: 50S ribosomal protein L36
+Macromolecule #32: 30S ribosomal protein S2
+Macromolecule #33: 30S ribosomal protein S3
+Macromolecule #34: 30S ribosomal protein S4
+Macromolecule #35: 30S ribosomal protein S5
+Macromolecule #36: 30S ribosomal protein S6
+Macromolecule #37: 30S ribosomal protein S7
+Macromolecule #38: 30S ribosomal protein S8
+Macromolecule #39: 30S ribosomal protein S9
+Macromolecule #40: 30S ribosomal protein S10
+Macromolecule #41: 30S ribosomal protein S11
+Macromolecule #42: 30S ribosomal protein S12
+Macromolecule #43: 30S ribosomal protein S13
+Macromolecule #44: 30S ribosomal protein S14
+Macromolecule #45: 30S ribosomal protein S15
+Macromolecule #46: 30S ribosomal protein S16
+Macromolecule #47: 30S ribosomal protein S17
+Macromolecule #48: 30S ribosomal protein S18
+Macromolecule #49: 30S ribosomal protein S19
+Macromolecule #50: 30S ribosomal protein S20
+Macromolecule #51: 30S ribosomal protein S21
+Macromolecule #59: GTP pyrophosphokinase
+Macromolecule #52: LSU rRNA
+Macromolecule #53: SSU rRNA
+Macromolecule #54: 5S rRNA
+Macromolecule #55: E-site tRNA(Phe)
+Macromolecule #56: P-site fMet-tRNA(fMet)
+Macromolecule #57: A/T tRNA(Phe)
+Macromolecule #58: mRNA
+Macromolecule #60: MAGNESIUM ION
+Macromolecule #61: ZINC ION
+Macromolecule #62: PAROMOMYCIN
+Macromolecule #63: METHIONINE
+Macromolecule #64: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK II / Details: Grids were blotted for 5 s. | ||||||||||||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: OTHER / Average electron dose: 35.0 e/Å2 Details: FEI Falcon III Images were collected in movie-mode at 30 frames per second |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 104478 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.8 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)