[English] 日本語
 Yorodumi
Yorodumi- PDB-7p7q: E. faecalis 70S ribosome bound by PoxtA-EQ2, high-resolution comb... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7p7q | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. faecalis 70S ribosome bound by PoxtA-EQ2, high-resolution combined volume | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | RIBOSOME / Enterococcus faecalis / PoxtA / ABCF / antibiotic resistance protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of translation / large ribosomal subunit / transferase activity / ribosomal small subunit biogenesis / ribosomal small subunit assembly / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding ...regulation of translation / large ribosomal subunit / transferase activity / ribosomal small subunit biogenesis / ribosomal small subunit assembly / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / RNA binding / zinc ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.4 Å | |||||||||
 Authors Authors | Crowe-McAuliffe, C. / Wilson, D.N. | |||||||||
| Funding support |  Germany, Germany,  Sweden, 2items Sweden, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural basis for PoxtA-mediated resistance to phenicol and oxazolidinone antibiotics. Authors: Caillan Crowe-McAuliffe / Victoriia Murina / Kathryn Jane Turnbull / Susanne Huch / Marje Kasari / Hiraku Takada / Lilit Nersisyan / Arnfinn Sundsfjord / Kristin Hegstad / Gemma C Atkinson / ...Authors: Caillan Crowe-McAuliffe / Victoriia Murina / Kathryn Jane Turnbull / Susanne Huch / Marje Kasari / Hiraku Takada / Lilit Nersisyan / Arnfinn Sundsfjord / Kristin Hegstad / Gemma C Atkinson / Vicent Pelechano / Daniel N Wilson / Vasili Hauryliuk /       Abstract: PoxtA and OptrA are ATP binding cassette (ABC) proteins of the F subtype (ABCF). They confer resistance to oxazolidinone and phenicol antibiotics, such as linezolid and chloramphenicol, which stall ...PoxtA and OptrA are ATP binding cassette (ABC) proteins of the F subtype (ABCF). They confer resistance to oxazolidinone and phenicol antibiotics, such as linezolid and chloramphenicol, which stall translating ribosomes when certain amino acids are present at a defined position in the nascent polypeptide chain. These proteins are often encoded on mobile genetic elements, facilitating their rapid spread amongst Gram-positive bacteria, and are thought to confer resistance by binding to the ribosome and dislodging the bound antibiotic. However, the mechanistic basis of this resistance remains unclear. Here we refine the PoxtA spectrum of action, demonstrate alleviation of linezolid-induced context-dependent translational stalling, and present cryo-electron microscopy structures of PoxtA in complex with the Enterococcus faecalis 70S ribosome. PoxtA perturbs the CCA-end of the P-site tRNA, causing it to shift by ∼4 Å out of the ribosome, corresponding to a register shift of approximately one amino acid for an attached nascent polypeptide chain. We postulate that the perturbation of the P-site tRNA by PoxtA thereby alters the conformation of the attached nascent chain to disrupt the drug binding site. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: Structural basis for PoxtA-mediated resistance to Phenicol and Oxazolidinone antibiotics Authors: Crowe-McAuliffe, C. / Murina, V. / Kasari, M. / Takada, H. / Turnbull, K.J. / Sundsfjord, A. / Hegstad, K. / Atkinson, G.C. / Wilson, D.N. / Hauryliuk, V. #2: Journal: mSphere / Year: 2018 Title: The Enterococcus Cassette Chromosome, a Genomic Variation Enabler in Enterococci. Authors: A Sivertsen / J Janice / T Pedersen / T M Wagner / J Hegstad / K Hegstad /  Abstract: has a highly variable genome prone to recombination and horizontal gene transfer. Here, we have identified a novel genetic island with an insertion locus and mobilization genes similar to those of ... has a highly variable genome prone to recombination and horizontal gene transfer. Here, we have identified a novel genetic island with an insertion locus and mobilization genes similar to those of staphylococcus cassette chromosome elements SCC This novel element termed the enterococcus cassette chromosome (ECC) element was located in the 3' region of and encoded large serine recombinases similar to SCC Horizontal transfer of an ECC element termed ECC:: containing a knock-in chloramphenicol resistance determinant occurred in the presence of a conjugative plasmid. We determined the ECC:: insertion site in the 3' region of in the recipient by long-read sequencing. ECC:: also mobilized by homologous recombination through sequence identity between flanking insertion sequence (IS) elements in ECC:: and the conjugative plasmid. The genes were found in 69 of 516 genomes in GenBank. Full-length ECC elements were retrieved from 32 of these genomes. ECCs were flanked by and sites of approximately 50 bp. The sequences were found by PCR and sequencing of circularized ECCs in three strains. The genes in ECCs contained an amalgam of common and rare genes. Taken together, our data imply that ECC elements act as hot spots for genetic exchange and contribute to the large variation of accessory genes found in is a bacterium found in a great variety of environments, ranging from the clinic as a nosocomial pathogen to natural habitats such as mammalian intestines, water, and soil. They are known to exchange genetic material through horizontal gene transfer and recombination, leading to great variability of accessory genes and aiding environmental adaptation. Identifying mobile genetic elements causing sequence variation is important to understand how genetic content variation occurs. Here, a novel genetic island, the enterococcus cassette chromosome, is shown to contain a wealth of genes, which may aid in adapting to new environments. The transmission mechanism involves the only two conserved genes within ECC, , large serine recombinases that insert ECC into the host genome similarly to SCC elements found in staphylococci. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7p7q.cif.gz 7p7q.cif.gz | 3.8 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7p7q.ent.gz pdb7p7q.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7p7q.json.gz 7p7q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7p7q_validation.pdf.gz 7p7q_validation.pdf.gz | 2.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7p7q_full_validation.pdf.gz 7p7q_full_validation.pdf.gz | 2.3 MB | Display | |
| Data in XML |  7p7q_validation.xml.gz 7p7q_validation.xml.gz | 227.9 KB | Display | |
| Data in CIF |  7p7q_validation.cif.gz 7p7q_validation.cif.gz | 412.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/p7/7p7q https://data.pdbj.org/pub/pdb/validation_reports/p7/7p7q ftp://data.pdbj.org/pub/pdb/validation_reports/p7/7p7q ftp://data.pdbj.org/pub/pdb/validation_reports/p7/7p7q | HTTPS FTP |
-Related structure data
| Related structure data |  13241MC  7p7rC  7p7sC  7p7tC  7p7uC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10764 (Title: Affinity-purified PoxtA in complex with 70S ribosomes from Enterococcus faecalis EMPIAR-10764 (Title: Affinity-purified PoxtA in complex with 70S ribosomes from Enterococcus faecalisData size: 321.8 Data #1: Unaligned multi-frame micrographs of PoxtA bound to 70S ribosome from Entrococcus faecalis [micrographs - multiframe]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 1 molecules 0
| #1: Protein | Mass: 67890.656 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
+50S ribosomal protein ... , 28 types, 28 molecules 12345678FGHIJKMNOPQRSTUVWXYZ
-RNA chain , 5 types, 5 molecules ABDab
| #10: RNA chain | Mass: 943422.312 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #11: RNA chain | Mass: 37433.188 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #12: RNA chain | Mass: 24811.795 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #33: RNA chain | Mass: 504278.750 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #34: RNA chain | Mass: 6219.850 Da / Num. of mol.: 1 / Source method: isolated from a natural source Details: Sequence unknown. Likely a mix of many different mRNAs. Source: (natural)  |
-30S ribosomal protein ... , 19 types, 19 molecules cdefghijklmnopqrstu
| #35: Protein | Mass: 29500.635 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #36: Protein | Mass: 24415.184 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #37: Protein | Mass: 23273.652 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #38: Protein | Mass: 17444.357 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #39: Protein | Mass: 11621.188 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #40: Protein | Mass: 17864.625 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #41: Protein | Mass: 14936.396 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #42: Protein | Mass: 14271.480 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #43: Protein | Mass: 11731.739 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #44: Protein | Mass: 13740.897 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #45: Protein | Mass: 15309.817 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #46: Protein | Mass: 13595.774 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #47: Protein | Mass: 7172.593 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #48: Protein | Mass: 10668.236 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #49: Protein | Mass: 10356.150 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #50: Protein | Mass: 10332.100 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #51: Protein | Mass: 9262.891 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #52: Protein | Mass: 10586.332 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #53: Protein | Mass: 8972.320 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Non-polymers , 7 types, 3960 molecules 




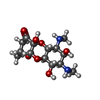







| #54: Chemical | | #55: Chemical | ChemComp-MG / #56: Chemical | ChemComp-K / #57: Chemical | ChemComp-ZN / #58: Chemical | #59: Chemical | ChemComp-SCM / | #60: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Units: MEGADALTONS / Experimental value: NO | ||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||
| Source (recombinant) | Organism:  | ||||||||||||||||||||||||||||
| Buffer solution | pH: 9 | ||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: Grid was prepared by four applications of elution fraction from affinity purification. | ||||||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/2 | ||||||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK II / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 278 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 165000 X / Nominal defocus max: 1500 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm / C2 aperture diameter: 70 µm |
| Image recording | Average exposure time: 5 sec. / Electron dose: 30.255 e/Å2 / Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 3639 |
| Image scans | Width: 3838 / Height: 3710 / Movie frames/image: 40 / Used frames/image: 1-40 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||
| Particle selection | Num. of particles selected: 203231 | ||||||||||||||||||||
| 3D reconstruction | Resolution: 2.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 112877 / Num. of class averages: 2 / Symmetry type: POINT |
 Movie
Movie Controller
Controller



















 PDBj
PDBj



































