[English] 日本語
 Yorodumi
Yorodumi- PDB-7o0w: Cryo-EM structure of the RC-dLH complex (model_1b) from Gemmatimo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7o0w | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the RC-dLH complex (model_1b) from Gemmatimonas phototrophica at 2.47 A | ||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Photosynthesis / reaction centre light harvesting complex / RC-dLH / RC-LH1 / carotenoid / quinone / bacteriochlorophyll a / Gemmatimonas phototrophica | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationorganelle inner membrane / plasma membrane light-harvesting complex / bacteriochlorophyll binding / photosynthetic electron transport in photosystem II / photosynthesis, light reaction / endomembrane system / electron transfer activity / iron ion binding / heme binding / metal ion binding ...organelle inner membrane / plasma membrane light-harvesting complex / bacteriochlorophyll binding / photosynthetic electron transport in photosystem II / photosynthesis, light reaction / endomembrane system / electron transfer activity / iron ion binding / heme binding / metal ion binding / membrane / plasma membrane Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  Gemmatimonas phototrophica (bacteria) Gemmatimonas phototrophica (bacteria) | ||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.47 Å | ||||||||||||||||||||||||
 Authors Authors | Qian, P. / Koblizek, M. | ||||||||||||||||||||||||
| Funding support |  Czech Republic, Czech Republic,  United Kingdom, European Union, 7items United Kingdom, European Union, 7items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: 2.4-Å structure of the double-ring photosystem. Authors: Pu Qian / Alastair T Gardiner / Ivana Šímová / Katerina Naydenova / Tristan I Croll / Philip J Jackson / Nupur / Miroslav Kloz / Petra Čubáková / Marek Kuzma / Yonghui Zeng / Pablo ...Authors: Pu Qian / Alastair T Gardiner / Ivana Šímová / Katerina Naydenova / Tristan I Croll / Philip J Jackson / Nupur / Miroslav Kloz / Petra Čubáková / Marek Kuzma / Yonghui Zeng / Pablo Castro-Hartmann / Bart van Knippenberg / Kenneth N Goldie / David Kaftan / Pavel Hrouzek / Jan Hájek / Jon Agirre / C Alistair Siebert / David Bína / Kasim Sader / Henning Stahlberg / Roman Sobotka / Christopher J Russo / Tomáš Polívka / C Neil Hunter / Michal Koblížek /     Abstract: Phototrophic Gemmatimonadetes evolved the ability to use solar energy following horizontal transfer of photosynthesis-related genes from an ancient phototrophic proteobacterium. The electron cryo- ...Phototrophic Gemmatimonadetes evolved the ability to use solar energy following horizontal transfer of photosynthesis-related genes from an ancient phototrophic proteobacterium. The electron cryo-microscopy structure of the photosystem at 2.4 Å reveals a unique, double-ring complex. Two unique membrane-extrinsic polypeptides, RC-S and RC-U, hold the central type 2 reaction center (RC) within an inner 16-subunit light-harvesting 1 (LH1) ring, which is encircled by an outer 24-subunit antenna ring (LHh) that adds light-gathering capacity. Femtosecond kinetics reveal the flow of energy within the RC-dLH complex, from the outer LHh ring to LH1 and then to the RC. This structural and functional study shows that has independently evolved its own compact, robust, and highly effective architecture for harvesting and trapping solar energy. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7o0w.cif.gz 7o0w.cif.gz | 1.2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7o0w.ent.gz pdb7o0w.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7o0w.json.gz 7o0w.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/o0/7o0w https://data.pdbj.org/pub/pdb/validation_reports/o0/7o0w ftp://data.pdbj.org/pub/pdb/validation_reports/o0/7o0w ftp://data.pdbj.org/pub/pdb/validation_reports/o0/7o0w | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  12681MC  7o0uC  7o0vC  7o0xC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10681 (Title: Movies of a photosynthetic reaction centre - double light-harvesting 1 complex from the bacterium G. phototrophica EMPIAR-10681 (Title: Movies of a photosynthetic reaction centre - double light-harvesting 1 complex from the bacterium G. phototrophicaData size: 12.1 TB Data #1: Unaligned multi-frame micrographs of RC-dLH1 complexes on HexAuFoil support [micrographs - multiframe]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 8 types, 31 molecules AAABACADAEAFAGAHAIAJAKALAMANAOAPAQARASATAUAVAWAXCC1C2H1H2LM
| #1: Protein | Mass: 6061.153 Da / Num. of mol.: 24 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) Gemmatimonas phototrophica (bacteria)#3: Protein | | Mass: 38457.609 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHR6 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHR6#4: Protein | | Mass: 21079.074 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) Gemmatimonas phototrophica (bacteria)#5: Protein | | Mass: 13656.403 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BK87 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BK87#6: Protein | | Mass: 7854.894 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BJ28 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BJ28#7: Protein | | Mass: 20022.566 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) Gemmatimonas phototrophica (bacteria)#8: Protein | | Mass: 30581.662 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHR2 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHR2#9: Protein | | Mass: 41154.074 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) Gemmatimonas phototrophica (bacteria) |
|---|
-Protein/peptide , 1 types, 40 molecules BABBBCBDBEBFBGBHBIBJBKBLBMBNBOBPBQBRBSBTBUBVBWBXbabbbcbdbebf...
| #2: Protein/peptide | Mass: 5084.809 Da / Num. of mol.: 40 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHS8 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHS8 |
|---|
-LHC domain-containing ... , 2 types, 16 molecules aaabacadaeafagahaiajakalamanaoap
| #10: Protein | Mass: 7695.042 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHS7 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHS7 |
|---|---|
| #11: Protein | Mass: 7723.052 Da / Num. of mol.: 15 / Source method: isolated from a natural source / Source: (natural)  Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHS7 Gemmatimonas phototrophica (bacteria) / References: UniProt: A0A143BHS7 |
-Sugars , 3 types, 127 molecules 
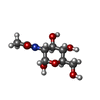

| #12: Polysaccharide | Type: oligosaccharide / Mass: 326.297 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source #14: Sugar | ChemComp-LMT / #19: Sugar | |
|---|
-Non-polymers , 14 types, 597 molecules 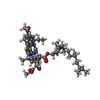



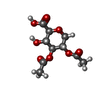
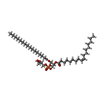





















| #13: Chemical | ChemComp-BCL / #15: Chemical | ChemComp-V7N / ( #16: Chemical | ChemComp-0V9 / ( #17: Chemical | ChemComp-HEC / #18: Chemical | #20: Chemical | ChemComp-PGW / ( | #21: Chemical | ChemComp-CD4 / ( #22: Chemical | #23: Chemical | #24: Chemical | ChemComp-FE / | #25: Chemical | ChemComp-CRT / | #26: Chemical | #27: Chemical | ChemComp-UYH / [( | #28: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: RC-dLH model_1b / Type: COMPLEX Details: Reaction centre light harvesting complex (model_1b) from Gemmatimonas phototrophic AP64 Entity ID: #1-#11 / Source: NATURAL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.800 MDa / Experimental value: NO | ||||||||||||
| Source (natural) | Organism:  Gemmatimonas phototrophica (bacteria) / Strain: AP64 Gemmatimonas phototrophica (bacteria) / Strain: AP64 | ||||||||||||
| Buffer solution | pH: 8 Details: The final purified complex is in 20mM Tris.Cl, 0.025 DDM, PH 8.0 buffer. | ||||||||||||
| Buffer component |
| ||||||||||||
| Specimen | Conc.: 6.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: The protein complex was isolated from photosynthetic membrane using detergent beta-DDM. After purification, concentrated protein sample solution was stored in LN before using. | ||||||||||||
| Specimen support | Grid material: GOLD / Grid type: Homemade | ||||||||||||
| Vitrification | Instrument: HOMEMADE PLUNGER / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K / Details: Talmon type |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 120000 X / Nominal defocus max: -2400 nm / Nominal defocus min: -800 nm / Cs: 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 1.2 sec. / Electron dose: 24.8 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 19865 Details: All movies were recorded from a HexAuFoil grid with a special EPU version, which can recognise 300 nm holes on the grid. |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1517482 | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.47 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 100616 / Algorithm: BACK PROJECTION / Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | B value: 30 / Protocol: FLEXIBLE FIT / Space: REAL / Details: ISOLDE incorporated in ChimeraX was used. | ||||||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 52.41 Å2 | ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj




























