+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7cbl | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the flagellar LP ring from Salmonella | |||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||
 Keywords Keywords | MOTOR PROTEIN / Flagella / LP ring / FlgH / FlgI | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationbacterial-type flagellum basal body, distal rod, L ring / bacterial-type flagellum basal body, distal rod, P ring / cytoskeletal motor activity / bacterial-type flagellum-dependent cell motility / cell outer membrane / outer membrane-bounded periplasmic space / structural molecule activity Similarity search - Function | |||||||||||||||||||||
| Biological species |  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) | |||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||||||||||||||
 Authors Authors | Tan, J.X. / Chang, S.H. / Wang, X.F. / Xu, C.H. / Zhou, Y. / Zhang, X. / Zhu, Y.Q. | |||||||||||||||||||||
| Funding support |  China, 6items China, 6items
| |||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: Structural basis of assembly and torque transmission of the bacterial flagellar motor. Authors: Jiaxing Tan / Xing Zhang / Xiaofei Wang / Caihuang Xu / Shenghai Chang / Hangjun Wu / Ting Wang / Huihui Liang / Haichun Gao / Yan Zhou / Yongqun Zhu /  Abstract: The bacterial flagellar motor is a supramolecular protein machine that drives rotation of the flagellum for motility, which is essential for bacterial survival in different environments and a key ...The bacterial flagellar motor is a supramolecular protein machine that drives rotation of the flagellum for motility, which is essential for bacterial survival in different environments and a key determinant of pathogenicity. The detailed structure of the flagellar motor remains unknown. Here we present an atomic-resolution cryoelectron microscopy (cryo-EM) structure of the bacterial flagellar motor complexed with the hook, consisting of 175 subunits with a molecular mass of approximately 6.3 MDa. The structure reveals that 10 peptides protruding from the MS ring with the FlgB and FliE subunits mediate torque transmission from the MS ring to the rod and overcome the symmetry mismatch between the rotational and helical structures in the motor. The LP ring contacts the distal rod and applies electrostatic forces to support its rotation and torque transmission to the hook. This work provides detailed molecular insights into the structure, assembly, and torque transmission mechanisms of the flagellar motor. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7cbl.cif.gz 7cbl.cif.gz | 2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7cbl.ent.gz pdb7cbl.ent.gz | 1.7 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7cbl.json.gz 7cbl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cb/7cbl https://data.pdbj.org/pub/pdb/validation_reports/cb/7cbl ftp://data.pdbj.org/pub/pdb/validation_reports/cb/7cbl ftp://data.pdbj.org/pub/pdb/validation_reports/cb/7cbl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  30335MC  7cbmC  7cg0C  7cg4C  7cg7C  7cgbC  7cgoC  7e80C  7e81C  7e82C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
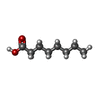
| #1: Protein | Mass: 24726.666 Da / Num. of mol.: 26 / Source method: isolated from a natural source Source: (natural)  Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) (bacteria) Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) (bacteria)References: UniProt: P0A1N8 #2: Protein | Mass: 38194.176 Da / Num. of mol.: 26 / Source method: isolated from a natural source Source: (natural)  Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) (bacteria) Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) (bacteria)References: UniProt: P15930 #3: Chemical | ChemComp-OCA / Has ligand of interest | N | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Flagellar hook-basal body / Type: COMPLEX / Entity ID: #1-#2 / Source: NATURAL | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) (bacteria) Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) (bacteria) | ||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 295 K / Details: blot for 6 seconds before plunging |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 47 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| Symmetry | Point symmetry: C26 (26 fold cyclic) |
| 3D reconstruction | Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 80548 / Symmetry type: POINT |
| Atomic model building | Protocol: AB INITIO MODEL / Space: REAL |
 Movie
Movie Controller
Controller

















 PDBj
PDBj