+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 4hbm | ||||||
|---|---|---|---|---|---|---|---|
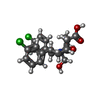
| タイトル | Ordering of the N Terminus of Human MDM2 by Small Molecule Inhibitors | ||||||
 要素 要素 | E3 ubiquitin-protein ligase Mdm2 | ||||||
 キーワード キーワード | ligase/ligase inhibitor / MDM2 / p53 / protein protein interaction / inhibitor / ligase-ligase inhibitor complex | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報cellular response to vitamin B1 / response to formaldehyde / response to water-immersion restraint stress / traversing start control point of mitotic cell cycle / response to ether / negative regulation of intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of signal transduction by p53 class mediator / fibroblast activation / atrial septum development / receptor serine/threonine kinase binding ...cellular response to vitamin B1 / response to formaldehyde / response to water-immersion restraint stress / traversing start control point of mitotic cell cycle / response to ether / negative regulation of intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of signal transduction by p53 class mediator / fibroblast activation / atrial septum development / receptor serine/threonine kinase binding / Trafficking of AMPA receptors / positive regulation of vascular associated smooth muscle cell migration / peroxisome proliferator activated receptor binding / SUMO transferase activity / negative regulation of protein processing / response to iron ion / response to steroid hormone / NEDD8 ligase activity / cellular response to peptide hormone stimulus / AKT phosphorylates targets in the cytosol / atrioventricular valve morphogenesis / ventricular septum development / endocardial cushion morphogenesis / positive regulation of muscle cell differentiation / cellular response to alkaloid / SUMOylation of ubiquitinylation proteins / regulation of protein catabolic process / blood vessel development / cardiac septum morphogenesis / ligase activity / Constitutive Signaling by AKT1 E17K in Cancer / response to magnesium ion / negative regulation of DNA damage response, signal transduction by p53 class mediator / SUMOylation of transcription factors / protein sumoylation / protein localization to nucleus / cellular response to UV-C / blood vessel remodeling / cellular response to estrogen stimulus / protein autoubiquitination / cellular response to actinomycin D / ribonucleoprotein complex binding / positive regulation of vascular associated smooth muscle cell proliferation / DNA damage response, signal transduction by p53 class mediator resulting in cell cycle arrest / transcription repressor complex / NPAS4 regulates expression of target genes / regulation of heart rate / positive regulation of mitotic cell cycle / positive regulation of protein export from nucleus / ubiquitin binding / proteolysis involved in protein catabolic process / response to cocaine / Stabilization of p53 / protein destabilization / Regulation of RUNX3 expression and activity / establishment of protein localization / RING-type E3 ubiquitin transferase / cellular response to growth factor stimulus / Oncogene Induced Senescence / Regulation of TP53 Activity through Methylation / cellular response to gamma radiation / response to toxic substance / cellular response to hydrogen peroxide / protein polyubiquitination / ubiquitin-protein transferase activity / disordered domain specific binding / endocytic vesicle membrane / ubiquitin protein ligase activity / p53 binding / Signaling by ALK fusions and activated point mutants / Regulation of TP53 Degradation / negative regulation of neuron projection development / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / 5S rRNA binding / cellular response to hypoxia / ubiquitin-dependent protein catabolic process / regulation of gene expression / protein-containing complex assembly / Oxidative Stress Induced Senescence / proteasome-mediated ubiquitin-dependent protein catabolic process / Regulation of TP53 Activity through Phosphorylation / amyloid fibril formation / regulation of cell cycle / Ub-specific processing proteases / protein ubiquitination / response to xenobiotic stimulus / protein domain specific binding / response to antibiotic / negative regulation of DNA-templated transcription / ubiquitin protein ligase binding / positive regulation of cell population proliferation / positive regulation of gene expression / negative regulation of apoptotic process / nucleolus / apoptotic process / negative regulation of transcription by RNA polymerase II / enzyme binding / protein-containing complex / zinc ion binding / nucleoplasm 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 1.9 Å 分子置換 / 解像度: 1.9 Å | ||||||
 データ登録者 データ登録者 | Huang, X. | ||||||
 引用 引用 |  ジャーナル: J.Am.Chem.Soc. / 年: 2012 ジャーナル: J.Am.Chem.Soc. / 年: 2012タイトル: Ordering of the N-terminus of human MDM2 by small molecule inhibitors. 著者: Michelsen, K. / Jordan, J.B. / Lewis, J. / Long, A.M. / Yang, E. / Rew, Y. / Zhou, J. / Yakowec, P. / Schnier, P.D. / Huang, X. / Poppe, L. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  4hbm.cif.gz 4hbm.cif.gz | 194.2 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb4hbm.ent.gz pdb4hbm.ent.gz | 156.1 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  4hbm.json.gz 4hbm.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  4hbm_validation.pdf.gz 4hbm_validation.pdf.gz | 3.4 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  4hbm_full_validation.pdf.gz 4hbm_full_validation.pdf.gz | 3.5 MB | 表示 | |
| XML形式データ |  4hbm_validation.xml.gz 4hbm_validation.xml.gz | 46.5 KB | 表示 | |
| CIF形式データ |  4hbm_validation.cif.gz 4hbm_validation.cif.gz | 63.3 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/hb/4hbm https://data.pdbj.org/pub/pdb/validation_reports/hb/4hbm ftp://data.pdbj.org/pub/pdb/validation_reports/hb/4hbm ftp://data.pdbj.org/pub/pdb/validation_reports/hb/4hbm | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| 7 | 
| ||||||||
| 8 | 
| ||||||||
| 9 | 
| ||||||||
| 10 | 
| ||||||||
| 11 | 
| ||||||||
| 12 | 
| ||||||||
| 単位格子 |
|
- 要素
要素
| #1: タンパク質 | 分子量: 13595.528 Da / 分子数: 8 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: MDM2 / 発現宿主: Homo sapiens (ヒト) / 遺伝子: MDM2 / 発現宿主:  参照: UniProt: Q00987, 合成酵素; C-N結合を形成; 酸-D-アミノ酸リガーゼ(ペプチド合成) #2: 化合物 | ChemComp-0Y7 / {( #3: 水 | ChemComp-HOH / | |
|---|
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 3.3 Å3/Da / 溶媒含有率: 62.76 % |
|---|---|
| 結晶化 | 温度: 277 K / 手法: 蒸気拡散法, ハンギングドロップ法 / pH: 5 詳細: 100 mM Citrate, pH 5.0 1.5-2.0 M (NH4)2SO4 , VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-データ収集
| 回折 | 平均測定温度: 100 K |
|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  APS APS  / ビームライン: 21-ID-F / ビームライン: 21-ID-F |
| 検出器 | タイプ: MARMOSAIC 225 mm CCD / 検出器: CCD |
| 放射 | プロトコル: SINGLE WAVELENGTH / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
| 放射波長 | 相対比: 1 |
| 反射 | 解像度: 1.9→50 Å / Num. all: 109869 / Num. obs: 413436 / % possible obs: 98.6 % / Observed criterion σ(I): -3 / Rmerge(I) obs: 0.057 |
| 反射 シェル | 解像度: 1.9→1.97 Å / Rmerge(I) obs: 0.493 / % possible all: 97.6 |
- 解析
解析
| ソフトウェア |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  分子置換 / 解像度: 1.9→50 Å / σ(F): 0 / 立体化学のターゲット値: Engh & Huber 分子置換 / 解像度: 1.9→50 Å / σ(F): 0 / 立体化学のターゲット値: Engh & Huber
| ||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 1.9→50 Å
|
 ムービー
ムービー コントローラー
コントローラー















 PDBj
PDBj